Creation of functional materials utilizing flurinated gases
Most fuorinated chemical products around us are synthesized using fuorine-based gases as raw materials. However, due to the diffculty in handling these gases, they are rarely used in academic research. As a result, research on fuorinated materials is largely limited to using commercially available products or a restricted set of fuorine-containing building blocks. Thanks to the strong industry-academia collaboration in our laboratory, we acquire expertise in handling fuorine-based gases, such as fuorine gas and tetrafuoroethylene gas. By utilizing reactions involving these fuorinated gases, we aim to develop organic and polymer materials endowed with the unique properties of fuorine while maintaining a high degree of fexibility in material design.
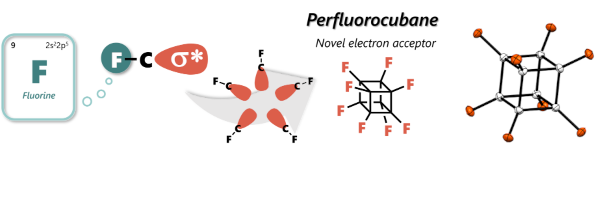
Synthesis and properties of highly-fluorinated cubanes.
Perfluoroubane, in which all carbon atoms of the cubic molecular framework of cubane are substituted with fluorine, is expected to accept an electron within its cubic skeleton. In our laboratory, we successfully synthesized perfluorocubane for the first time in the world using fluorine gas. Furthermore, we successfully confined the state in which an electron was encapsulated inside the structure. We aim to elucidate the unique properties of highly-fluorinated cubanes and develop new functional materials utilizing this molecule.
Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor
Sugiyama, M.; Akiyama, M.*; Yonezawa, Y.; Komaguchi, K.; Higashi, M.; Nozaki, K.; Okazoe, T. Science, 2022, 377, 756–759.
Exceptionally Short Tetracoordinated Carbon–Halogen Bonds in Hexafluorodihalocubanes
Sugiyama, M.; Uetake, Y.*; Miyagi, N.; Yoshida, M.; Nozaki, K.; Okazoe, T.; Akiyama, M.* J. Am. Chem. Soc., 2024, 146, 30686–30697.
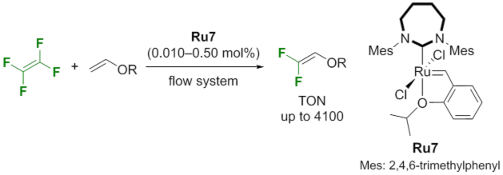
Development of novel catalysts and reactions for olefin metathesis using fluoroolefins.
Olefin metathesis is a reaction that rearranges the double bonds of olefins and was the subject of the 2005 Nobel Prize in Chemistry. However, the metathesis of olefins directly bonded to fluorine atoms has long been considered challenging. In our laboratory, we are developing olefin metathesis reactions using tetrafluoroethylene, which is relatively inexpensive in industrial applications. We have discovered that a ruthenium complex with a seven-membered ring N-heterocyclic carbene ligand (Ru7) exhibits catalytic activity more than 100 times higher than that of conventional catalysts.
The 1,1-difluoroethylene derivatives produced through this process are known to be useful as environmentally friendly refrigerants, polymer precursors, and pharmaceutical intermediates. Therefore, this research is expected to contribute to the development of new functional materials.
Highly Active Cross Metathesis of Tetrafluoroethylene with a Seven-membered NHC-Ruthenium Catalyst
K. Mori, M. Akiyama*, K. Inada,Y. Imamura, Y. Ishibashi, Y. Takahira, K. Nozaki*, T. Okazoe, J. Am. Chem. Soc., 2021, 143, 20980–20987.
Akiyama, M.*; Amabe, Y.; Sugiyama, M.; Sugiyama, K.; Gatzenmeier, T.; Okazoe, T. J. Am. Chem. Soc., 2024, in press.
Development of novel electrophilic fluorinating agents for selective fluorination and facile functionalization of small organic molecules.
Organofluorine compounds play a crucial role in the fields of pharmaceuticals and agrochemicals. In the development of fluorine-containing pharmaceuticals and agrochemicals, the chemical, regio-, and stereoselective introduction of fluorine atoms is essential. In our laboratory, we are developing new electrophilic fluorinating agents using fluorine gas to enable the selective incorporation of fluorine atoms.


Cover Gallery
Bench-Stable Electrophilic Fluorinating Reagents for Highly Selective Mono- and Difluorination of Silyl Enol Ethers
A. Adachi, K. Aikawa, Y. Ishibashi, K. Nozaki, T. Okazoe Chem. Eur. J., 2021, 27, 11919–11925.
Adachi, A.; Hashimoto, T.*; Aikawa, K.*; Nozaki, K.; Okazoe, T. Org. Chem. Front. 2023, 10, 5362–5368.
In addition, focusing on the use of N–F bond-containing fluorinating reagents as nitrogen atom transfer reagents, we have developed N–F reagents with tunable substituents on nitrogen, designed to enhance reactivity, facile deprotection, and enable functionalization. We have also reported on their reactivity and applications. N-Fluorobenzenesulfonimide (NFSI) analogs with deprotectable substituents: Synthesis of β-fluoroamines via catalytic aminofluorination of styrenes
Ito, Y.; Adachi, A.; Aikawa, K.*; Nozaki, K.; Okazoe, T. Chem. Commun. 2023, 59, 9195–9198.
Y. Oe, R. Yoshida, A. Tanaka, A. Adachi, Y. Ishibashi, T. Okazoe, K. Aikawa*, T. Hashimoto*, J. Am. Chem. Soc., 2022, 144, 2107–2113.
This electrophilic fluorinating reagent developed in our laboratory, N-fluoro-N-(fluorosulfonyl)carbamate: NFC, has been commercialized by Tokyo Chemical Industry (TCI Inc.). (Currently unavailable)
