Research in the Nozaki Group
Our research focuses on organic chemistry. The goal of our research is to discover, develop, and understand new reactions mediated by homogeneous catalysis for organic and polymer synthesis. Our interests further extend to the functions of new materials created by our original methods. We pursue our interests for both the beauty of science and to positively impact society.
The followings are our research topics in recent years:
1-1. Chemical Synthesis Using Carbon Dioxide as a Starting Material
CO2 is an earth-abundant and inexpensive carbon resource, thus a desirable C1 source. Our group has been engaged in polymer synthesis or versatile chemical synthesis using CO2 as a starting material.
1-1-1. Polymer Synthesis Using Carbon Dioxide as a Starting Material
Since CO2 is the most stable state of carbon, external energy is required to convert it into other substances. In polymer syntheses, CO2 can be integrated into polymers by copolymerization with highly reactive monomers.
Our group successfully synthesized various polymers derived from CO2/alkenes by homo- or co-polymerization of EVP lactone made from CO2 and butadiene. Homopolymerization (radical polymerization) of EVP afforded a polymer with high CO2 content (up to 29 wt%).(1) The obtained polymer showed unique properties, such as high glass transition temperature and decomposition temperature, which are different from those of other reported polymers made from CO2. The main chain structure of this polymer can be modified reversibly by the reaction with water or amines.(2) This result suggests that the physical property of EVP polymer can be tuned reversibly.
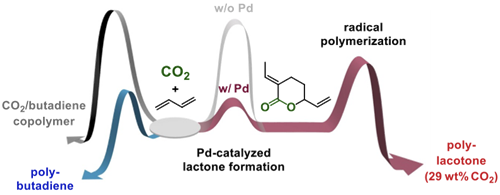
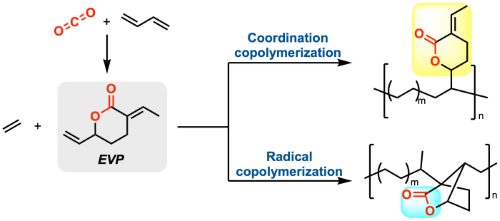
Our group developed copolymerization of EVP with other olefin monomers as well. Copolymerization of EVP with ethylene was achieved by both coordination polymerization with a Pd catalyst and radical polymerization.(3) The structure of the resulting polymer was dependent on the polymerization methods: A polymer with lactones on the side chain was obtained by coordination polymerization, whereas a polymer with a lactone main chain was acquired by radical polymerization. Our group also reported copolymerization of EVP with methyl acrylate, styrene, and vinyl acetate by radical polymerization to tune the physical properties of the resulting polymer.(4)
Recent Publication
(1) Nakano, R.; Ito, S.; Nozaki, K. Nat. Chem. 2014, 6, 325–331. 10.1038/nchem.1882
(2) Moon, S.; Masada, K.; Nozaki, K. J. Am. Chem. Soc. 2019, 141, 10938–10942. 10.1021/jacs.9b03205
(3) Tang, S.; Zhao, Y.; Nozaki, K. J. Am. Chem. Soc. 2021, 143, 17953–17957. 10.1021/jacs.1c08578
(4) Hill, M.; Tang, S.; Masada, K.; Hirooka, Y.; Nozaki, K. Macromolecules 2022, 55, 3311–3316. 10.1021/acs.macromol.1c02503
1-1-2. Versatile Chemical Synthesis Using Carbon Dioxide as a Starting Material
Formic acid is an important chemical produced in over 700,000 t per year for such as preservatives and insecticides. Current industrial production of formic acid generally employs carbon monoxide and water. Our group utilized the Ir-PNP hydride complex in catalytic hydrogenation of CO2 and recorded a TOF of 150,000 h–1 (the highest when published) and a TON of 3,500,000 (the highest still now). We also developed highly active catalysis to get formic acid using an organic base.(1,2)
In addition, we are searching for various methods to prepare valuable chemicals via CO2 reduction. For example, we reported the synthesis of α-hydroxy acids via reductive coupling of an aldehyde and CO2 mediated by a Cu complex.(3)
Recent Publication
(1) Aoki, W.; Wattanabinin, N.; Kusumoto, S.; Nozaki, K. Bull. Chem. Soc. Jpn. 2016, 89, 113–124. 10.1246/bcsj.20150311
(2) Takaoka, S.; Eizawa, A.; Kusumoto, S.; Nakajima, K.; Nishibayashi, Y.; Nozaki, K. Organometallics 2018, 37, 3001–3009. 10.1021/acs.organomet.8b00377
(3) Masada, K.; Kusumoto, S.; Nozaki, K. Org. Lett. 2020, 22, 4922–4926. 10.1021/acs.orglett.0c00995
1-2. Chemical Synthesis Using Carbon Monoxide as a Starting Material
The hydroformylation reaction producing aldehydes from alkenes, carbon monoxide and hydrogen, is one of the most important reactions in industry. We have been engaged in developing new transition metal catalysts to improve the catalytic activity and regioselectivity of the hydroformylation reaction.
In general, group 9 metals such as rhodium or cobalt are used as catalysts for hydroformylation reactions. In our research, we succeeded in developing ruthenium-catalyzed hydroformylation, combined with a bulky phosphite ligand, to accomplish selective production of linear aldehydes from 1-alkenes.(1) Moreover, we recently reported the branch-selective hydroformylation of 1-alkene using rhodium and tridentate N-Triphos ligand.(2)
We also developed retro-hydroformylation reaction, which has rarely been studied before.(3) In the presence of an iridium catalyst, long-chain aliphatic aldehydes were converted into the corresponding alkenes, carbon monoxide, and dihydrogen in high yield. This reaction might open the door for the utilization of renewable resources as feedstocks.
In industry, aliphatic alcohols are generally produced by two step procedures of olefin hydroformylation and subsequent hydrogenation. In this context, we developed an effective one-pot process to produce linear alcohols from 1-alkenes by combining a rhodium-catalyzed hydroformylation and a ruthenium-catalyzed hydrogenation in the same reaction vessel.(4-6) It is expected to contribute to improvement of petrochemical processes. We are currently searching for more active processes by combination with heterogeneous catalysts.
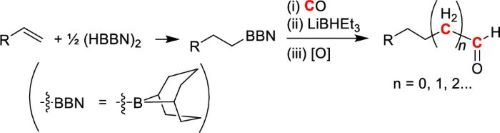
Recently, we also found heavy-metal-free homologation of a carbon chain from alkylborane, which is made from alkene and hydroborane, with CO and a hydride source.(7)
Recent Publication
(1) Takahashi, K.; Yamashita, M.; Tanaka, Y.; Nozaki, K. Angew. Chem. Int. Ed. 2012, 51, 4283–4387. 10.1002/anie.201108396
(2) Phanopoulos, A.; Nozaki, K. ACS Catal. 2018, 8, 5799–5809. 10.1021/acscatal.8b00566
(3) Kusumoto, S.; Tatsuki, T.; Nozaki, K. Angew. Chem. Int. Ed. 2015, 54, 8458–8461. 10.1002/anie.201503620
(4) Takahashi, K.; Yamashita, M.; Ichihara, T.; Nakano, K.; Nozaki, K. Angew. Chem. Int. Ed. 2010, 49, 4488–4490. 10.1002/anie.201001327
(5) Takahashi, K.; Yamashita, M.; Nozaki, K. J. Am. Chem. Soc. 2012, 134, 18746–18757. 10.1021/ja307998h
(6) Yuki, Y.; Takahashi, K.; Tanaka, Y.; Nozaki, K. J. Am. Chem. Soc. 2013, 135, 17393–17400. 10.1021/ja407523j
(7) Phanopoulos, A.; Pal, S.; Kawakami, T.; Nozaki, K. J. Am. Chem. Soc. 2020, 142, 14064–14068. 10.1021/jacs.0c06580
1-3. Bond Cleavage/Formation by Metal–Ligand Cooperation
In contrast to classical oxidative addition/reductive elimination directly at a metal center, metal–ligand cooperation, where metal and ligand are involved in the cleavage/formation of one bond at once, has been attracting attention. The cooperativity has enabled unique reactivity and selectivity, which cannot be achieved only by a metal center. Our group has investigated the catalytic activity of transition-metal complexes bearing redox-active cyclopentadienone–hydroxycyclopentadienyl ligands for various bond-cleavage and bond-forming reactions. Recently, we developed the first direct and acceptorless dehydrogenation of saturated hydrocarbons,(1,2) hydrogenolysis of C–O bonds,(3) and dehydrogenation of amine–borane(4) by employing cyclopentadienyl iridium/rhodium hydride complexes. These transformations can be applied to synthetic organic chemistry, pharmaceutical chemistry, and the refinery of biomaterials. Indeed, we demonstrated the catalytic cleavage of C–O, and C–C bonds in a lignin-model compound by cyclopentadienone metal complexes.(5) We are currently trying to apply the reactivity to resin degradation.
Whereas the reactions above are based on cleavage or formation of an H–H bond, recently, metal–ligand cooperative cleavage and formation of various chemical bonds have been extensively studied.(6) Our group has reported the first metal–ligand cooperative C–H bond heterolytic reductive elimination from the hydroxycyclopentadienyl dimethylplatinum(IV) complex.(7) Moreover, we achieved the reverse reaction, heterolytic oxidative addition by a precisely designed iridium complex bearing an electron-deficient cyclopentadienone ligand. The catalyst was active to cleave a C–H bond of methane, which would lead to the utilization of simple alkanes.(8)
The cyclopentadienone iridium(I) complex was additionally found to cleave B–H/Si–H bonds in hydroboranes and hydrosilanes into the H+ on the ligand and the B–/Si– on the metal, which can be regarded as umpolung of the B–H/Si–H bond.(9,10) The hydroxycyclopentadienyl (triphenylsilyl)iridium complex subsequently gives diphenylsilylene iridium complex by releasing benzene via intramolecular protonolysis.(10)
Recent Publication
(1) Kusumoto, S.; Akiyama, M.; Nozaki, K. J. Am. Chem. Soc. 2013, 135, 18726–18729. 10.1021/ja409672w
(2) Ando, H.; Kusumoto, S.; Wu, W.; Nozaki, K. Organometallics 2017, 36, 2317–2322. 10.1021/acs.organomet.7b00245
(3) Kusumoto, S; Nozaki, K. Nat. Commun. 2015, 6, 6296. 10.1038/ncomms7296
(4) Pal, S.; Kusumoto, S.; Nozaki, K. Organometallics 2018, 37, 906–914. 10.1021/acs.organomet.7b00889
(5) Kusumoto, S.; Kishino, M.; Nozaki, K. Chem. Lett. 2020, 49, 477–480. 10.1246/cl.200037
(6) Higashi, T.; Kusumoto, S.; Nozaki, K. Chem. Rev. 2019, 119, 10393–10402. 10.1021/acs.chemrev.9b00262
(7) Higashi, T.; Ando, H.; Kusumoto, S.; Nozaki, K. J. Am. Chem. Soc. 2019, 141, 2247–2250. 10.1021/jacs.8b13829
(8) Higashi, T.; Kusumoto, S.; Nozaki, K. J. Am. Chem. Soc. 2021, 143, 12999–13004. 10.1021/jacs.1c06714
(9) Higashi, T.; Kusumoto, S.; Nozaki, K. Angew. Chem. Int. Ed. 2021, 60, 2844–2848. 10.1002/anie.202011322
(10) Higashi, T.; Kusumoto, S.; Nozaki, K. Organometallics 2022, 41, 659–665. 10.1021/acs.organomet.2c00037
2-1. Synthesis of Novel Highly Functional Polymers
Incorporating polar functional groups into the main chain of polyolefins can improve their hydrophilic properties, such as adhesion, dyeability, and colorability, drastically expanding the range of applications. To this end, we focus on the copolymerization of olefins with polar monomers as one of the most effective methods to synthesize functionalized polyolefins. We have thus far achieved the coordination-insertion copolymerization of ethylene and various polar monomers catalyzed by palladium catalysts carrying a phosphine-sulfonate ligand (1) but for industrial applications, further improvement of activity and molecular weight of the polymer is desired. Recently, we investigated the steric effect of substituents on phosphine-sulfonate ligands systematically to find catalysts affording copolymers with exceptionally high molecular weights than previous catalyst systems.(2) Furthermore, we applied machine learning to the design of catalysts and realized palladium complexes ligated by phosphine-sulfonate, achieving high activity in the copolymerization of ethylene and methyl acrylate.(3)
Based on a hypothesis that the use of unsymmetric bidentate ligands is the key to the successful coordination-insertion copolymerization of ethylene with polar monomers, we have developed palladium catalysts bearing bisphosphine monoxide (BPMO) ligands(4,5) and bidentate ligands containing N-heterocyclic carbene (NHC) such as imidazo[1,5-a]quinolin-9-olate-1-ylidene (IzQO) ligands(6-10) as novel effective polymerization catalysts. In addition, we utilized economical nickel catalysts as an alternative to expensive palladium catalysts in the copolymerization of polar monomers with olefins.(11-15)
Using the palladium catalysts bearing bidentate ligands mentioned above, we reported the copolymerization of ethylene with polar monomers such as 1,1-disubstituted ethylene or carbenes,(16-18) which have never been reported previously.
We have achieved coordination-insertion copolymerization of propylene with polar monomers, which is much more challenging than ethylene/polar monomer copolymerization using IzQO ligand.(6) Furthermore, palladium(19) and nickel(13) catalysts, which have chiral menthyl groups on the phosphorus atom, produced moderately stereoselective copolymers. Recently, we also found that palladium catalysts bearing P-stereogenic phosphine-sulfonate and BPMO ligands, in which two different-sized substituents are attached, could afford highly isotactic copolymer of propylene and polar monomers.(20)
Recent Publication
(1) Ito, S.; Kanazawa, M.; Munakata, K.; Kuroda, J.; Okumura, Y.; Nozaki, K. J. Am. Chem. Soc. 2011, 133, 1232–1235. 10.1021/ja1092216
(2) Ota, Y.; Ito, S.; Kuroda, J.; Okumura, Y.; Nozaki, K. J. Am. Chem. Soc. 2014, 136, 11898–11901. 10.1021/ja505558e
(3) Akita, S.; Guo, J -Y.; Seidel, F.; Sigman, M.; Nozaki, K. Organometallics 2022, in press. 10.1021/acs.organomet.2c00066
(4) Mitsushige, Y.; Carrow, B. P.; Ito, S.; Nozaki, K. Chem. Sci. 2016, 7, 737–744. 10.1039/C5SC03361F
(5) Mitsushige, Y.; Yasuda, H.; Carrow, B.; Ito, S.; Kobayashi, M.; Tayano, T.; Watanabe, Y.; Okuno, Y.; Hayashi, S.; Kuroda, J.; Okumura, Y.; Nozaki, K. ACS Macro Lett. 2018, 7, 305–311. 10.1021/acsmacrolett.8b00034
(6) Nakano, R.; Nozaki, K. J. Am. Chem. Soc. 2015, 137, 10934–10937. 10.1021/jacs.5b06948
(7) Tao, W.; Akita, S.; Nakano, R.; Ito, S.; Hoshimoto, Y.; Ogoshi, S.; Nozaki, K. Chem. Commun. 2017, 53, 2630–2633. 10.1039/C7CC00002B
(8) Akita, S.; Nakano, R.; Ito, S.; Nozaki, K. Organometallics 2018, 37, 2286–2296. 10.1021/acs.organomet.8b00263
(9) Tao, W.; Wang, X.; Ito, S.; Nozaki, K. J. Polym. Sci. Part A: Polym. Chem. 2019, 57, 474–477. 10.1002/pola.29270
(10) Akita, S.; Nozaki, K. Polym. J. 2021, 53, 1057–1060. 10.1038/s41428-021-00500-3
(11) Ito, S.; Ota, Y.; Nozaki, K.; Dalton Trans. 2012, 41, 13807–13809. 10.1039/C2DT31771K
(12)Tao, W.; Nakano, R.; Ito, S.; Nozaki, K. Angew. Chem. Int. Ed. 2016, 55, 7505–7509. 10.1002/anie.201600819
(13) Konishi, Y.; Tao, W.; Yasuda, H.; Ito, S.; Oishi, Y.; Ohtaki, H.; Tanna, A.; Tayano, T.; Nozaki, K. ACS Macro Lett. 2018, 7, 213–217. 10.1021/acsmacrolett.7b00904
(14) Jung, J.; Yasuda, H.; Nozaki, K. Macromolecules 2020, 53, 2547–2556. 10.1021/acs.macromol.0c00183
(15) Seidel, F. W.; Nozaki, K. Angew. Chem. Int. Ed. 2022, 62, e202111691. 10.1002/anie.202111691
(16) Wang, X.; Seidel, F. W.; Nozaki, K. Angew. Chem. Int. Ed. 2019, 58, 12955–12959. 10.1002/anie.201906990
(17) Wang, X.; Nozaki, K. J. Am. Chem. Soc. 2018, 140, 15635–15640. 10.1021/jacs.8b10335
(18) Yasuda, H.; Nakano, R.; Ito, S.; Nozaki, K. J. Am. Chem. Soc. 2018, 140, 1876–1883. 10.1021/jacs.7b12593
(19) Ota, Y.; Ito, S.; Kobayashi, M.; Kitade, S.; Sakata, K.; Tayano, T.; Nozaki, K. Angew. Chem. Int. Ed. 2016, 55, 7505–7509. 10.1002/anie.201600819
(20) Seidel, F. W.; Tomizawa, I.; Nozaki, K. Angew. Chem. Int. Ed. 2020, 59, 22591–22601. 10.1002/anie.202009027
2-2. Polymer Degradation

Considering the environmental impact of plastic waste, we are engaged in developing degradable plastics. We have succeeded in synthesizing a polymer in which carbon monoxide is randomly incorporated into the main polyethylene chain, employing metal carbonyl as a carbon monoxide source. The obtained polymer was found to be partially degraded upon UV irradiation.(1) We are currently searching catalytic systems for degrading plastics.
Recent Publication
(1) Tang, S.; Seidel, F. W.; Nozaki, K. Angew. Chem. Int. Ed. 2021, 60, 26506–26510. 10.1002/anie.202110957
Beautiful molecules —those electronic structures are controlled precisely— show novel properties. In this chapter, we will introduce “beautiful molecules” recently we synthesized.
3-1. Synthesis of Novel Ligands with Heterobenzene
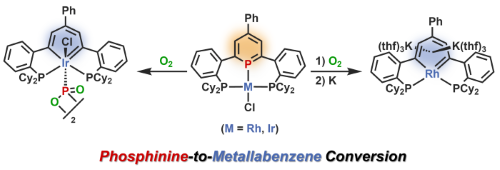
Phosphinine (phosphabenzene) is a heteroaromatic compound where a CH group of benzene is substituted by a phosphorus atom. Phosphinine shows interesting properties such as strong electron-withdrawing character and reactivity of sp2-hybridized phosphorus. Our group reported a novel phosphinine pincer ligand with two phosphine pendants.(1) When an iridium complex and a rhodium complex of the pincer ligand were treated with oxygen gas, metallabenzenes were obtained via the substitution of the phosphorus atom on the phosphinine ring by the metal fragments. Notably, this is the first synthesis of rhodabenzene.
Recent Publication
(1) Masada, K.; Kusumoto, S.; Nozaki, K. Angew. Chem. Int. Ed. 2022, 61, e202117096. 10.1002/anie.202117096
3-2. Synthesis of Triptycene Skeletal Molecules
Triptycene, where three benzene rings are fused at 120 degrees, has unique properties due to the rigid 3-fold symmetry structure. We recently synthesized triptycenemonohydroquinonedibenzoquinone (HQ)1(BQ)2, in which one hydroquinone and two benzoquinone unit(s) coexist in the triptycene backbone, by comproportionation of triptycenetrihydroquinone (HQ)3 and triptycenetribenzoquinone (BQ)3. The crystal structure of (HQ)1(BQ)2 exhibits a hydrogen bonding network while it undergoes disproportionation in the solution.(1)
Recent Publication
(1) Takemasa, Y.; Nozaki, K. J. Org. Chem. 2022, 87, 1502–1506. 10.1021/acs.joc.1c01683
3-3. Synthesis of Helicene Derivatives
Helicenes, ortho-fused aromatic rings in a helical fashion, show characteristic properties derived from their quasi-planarity and helical chirality. We previously synthesized helicene analogs with a five-membered ring containing a heteroatom, such as nitrogen, oxygen, phosphorus, and silicon. They were found to show various properties depending on the included heteroatom.(1-4) Recently, we synthesized spiro-double sila[7]helicene, in which two sila[7]helicenes were connected perpendicularly by the shared spiro Si atom. The efficient overlapping of orbitals (spiro-conjugation) lowers the LUMO level due to the spiro-double helicene geometry.(5) We are trying to connect two or three helicene moieties by a transition metal atom since we demonstrated the introduction of six phenyl rings in a transition metal center.(6)
Recent Publication
(1) Nakano, K.; Hidehira, Y.; Takahashi, K.; Hiyama, T.; Nozaki, K. Angew. Chem. Int. Ed. 2005, 44, 7136–7138. 10.1002/anie.200502855
(2) Nakano, K.; Oyama, H.; Nishimura, Y.; Nakasako, S.; Nozaki, K. Angew. Chem. Int. Ed. 2012, 51, 695–699. 10.1002/anie.201106157
(3) Oyama, H.; Nakano, K.; Harada, R.; Kuroda, R; Naito, M.; Nobusawa, K.; Nozaki, K. Org. Lett. 2013, 15, 2104–2107. 10.1021/ol4005036
(4) Oyama, H.; Akiyama, M.; Nakano, K.; Naito, M.; Nobusawa, K.; Nozaki, K. Org. Lett. 2016, 18, 3654–3657. 10.1021/acs.orglett.6b01708
(5) Terada, N.; Uematsu, K.; Higuchi, R.; Tokimaru, Y.; Sato, Y.; Nakano, K.; Nozaki, K. Chem. Eur. J. 2021, 27, 9342–9349. 10.1002/chem.202100385
(6) Iwasaki, T.; Hirooka, Y.; Takaya, H.; Honma, T.; Nozaki, K. Organometallics 2021, 40, 2489–2495. 10.1021/acs.organomet.1c00248
3-4. Main Group Element Chemistry and Structural Organic Chemistry
Low-valent main group element compounds are of interest for their unique electronic structures and the resulting characteristic molecular structures and reactivities. We are mainly focusing on low-valent carbon species, “carbene (C(II))”(1) and “carbone (C(0))”,(2) from a perspective of structural organic/coordination chemistry. We will expand the project from carbon to other main group elements to reveal their unpredecented properties.
Recent Publication
(1) Shibutani, Y.; Kusumoto, S.; Nozaki, K. J. Am. Chem. Soc. 2023, 145, 16186–16192.
(2) Saito, K.; Kusumoto, S.; Nozaki, K. Chem. Eur. J. 2023, e202302060.
Heterogeneous catalysts can be easily recovered and reused, compared to homogeneous catalysts that dissolve in the reaction solution. However, homogeneous catalysts are still widely used in synthetic organic chemistry because it is difficult to precisely design the structure of the active site of the heterogeneous catalysts. We are creating “structurally and mechanistically well-defined heterogeneous catalysts,” which possess the advantages of both heterogeneous and homogeneous catalysts to develop challenging synthetic organic reactions.
4-1. Selective Hydrogenolysis of Phenols to Aromatic Hydrocarbons
Selective hydrogenolysis of phenols to aromatic hydrocarbons is an essential reaction for biomass conversion and fine chemical synthesis. We have developed a platinum nanoparticle catalyst possessing aluminum metaphosphate (Al(PO3)3) as support which selectively catalyzed hydrogenolysis of a wide range of phenols to the corresponding aromatic hydrocarbons under a milder condition than conventional catalytic systems.(1) Mechanistic studies suggested that the cooperativity of the support and platinum nanoparticles was the key for the selectivity.
Recent Publication
(1) Jin, X.; Tsukimura, R.; Aihara, T.; Miura, H.; Shishido, T.; Nozaki, K. Nat. Catal. 2021, 4, 312–321. 10.1038/s41929-021-00598-x