RESEARCH
Research 1 Development of highly efficient and novel molecular transformations
Although liquid-phase organic reactions have been widely utilized from laboratory-level chemical synthesis to industrial production of medicines, agricultural chemicals, and fine chemicals, there is still room for improvement in terms of their greenness, resource utilization efficiency, and energy efficiency. For example, conversions of various organic substrates to oxygen-containing compounds and oxidative functional group transformations represented by dehydrogenation reactions still use superstoichiometric amounts of oxidants such as high valent chromium or manganese reagents in some cases. In addition, these antiquated methods also have serious problems such as the processing of metal salt wastes.
We have been engaged in research on the development of high-performance heterogeneous catalysts for highly efficient liquid-phase organic reactions, especially for catalytic oxidation reactions. We have introduced new molecular design concepts in the field of heterogeneous catalysts and created original nanostructured catalysts (including molecular and crystalline nano oxides, nano hydroxides, metal nanoparticles, and alloys) from our unique viewpoints and methods (Fig. 1.1).
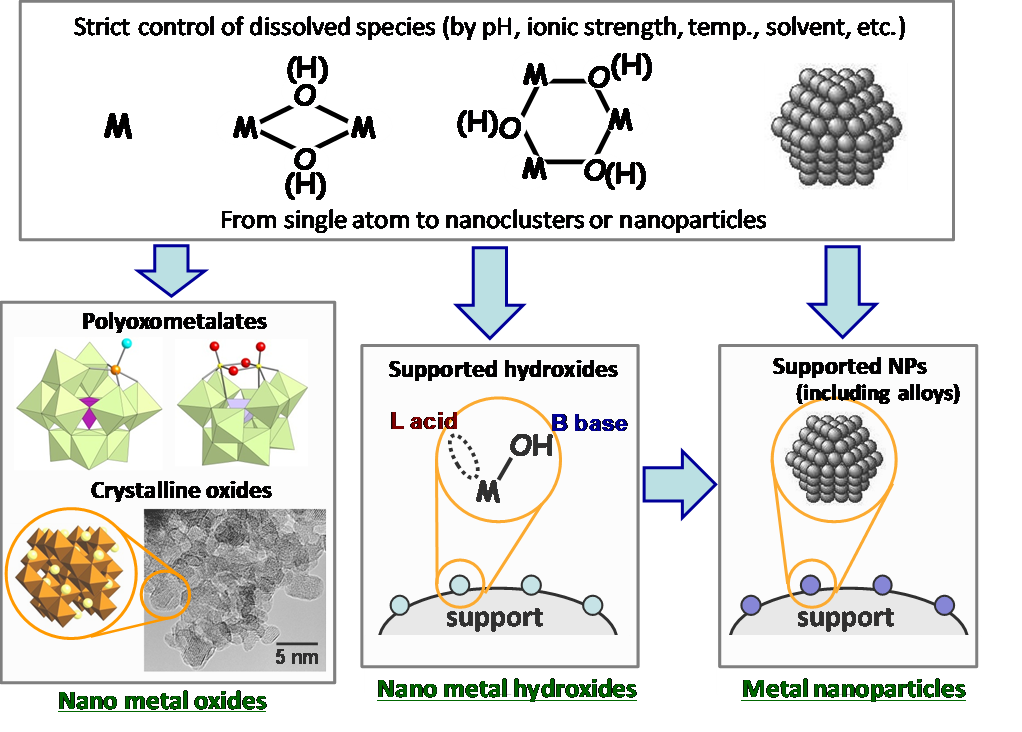
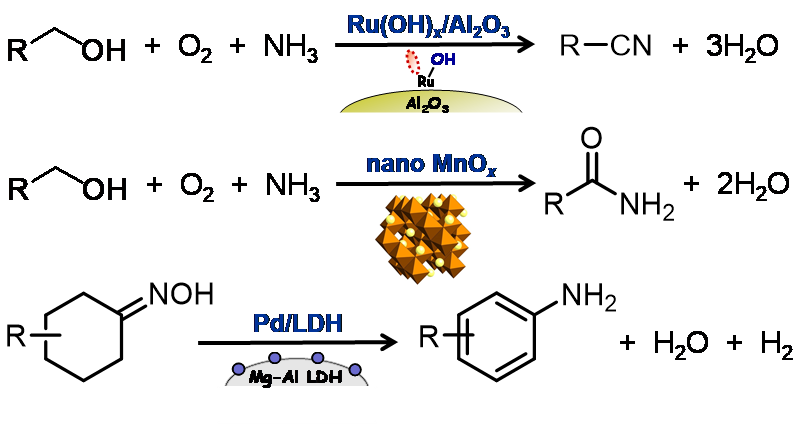
We have succeeded in developing several environmentally conscious highly efficient liquid-phase functional group transformations (including one-pot synthesis) using our nanostructured catalysts; for example, oxygenation (epoxidation) with high efficiencies of hydrogen peroxide utilization (>99%) by polyoxometalate-based catalysts[1.1], ammoxidation utilizing concerted alcohol activation by Bronsted base and Lewis acid in nano ruthenium hydroxide[1.2] (Fig. 1.2, top), amidation of alcohols, alkylarenes, and amines using the oxidation and water activation abilities of crystalline nanomanganese oxide[1.3] (Fig. 1.2, middle). In addition, we have newly discovered the specific dehydrogenation abilities of palladium, gold or their alloy nanoparticles. Utilizing their specific dehydrogenation abilities, various new aromatic ring formation reactions (dehydrogenative aromatization), such as phenol synthesis from cyclohexanols[1.4], selective arylamine synthesis (anilines vs diarylamines vs triarylamines)[1.5], aniline synthesis from oximes[1.6] (Fig. 1.2, bottom), and selective azobenzene synthesis[1.7], have been realized. Recently, we have found that nickel, inexpensive non-precious metal, can efficiently catalyze various dehydrogenative aromatization reactions by suitably preparing nanoparticle catalysts[1.8].
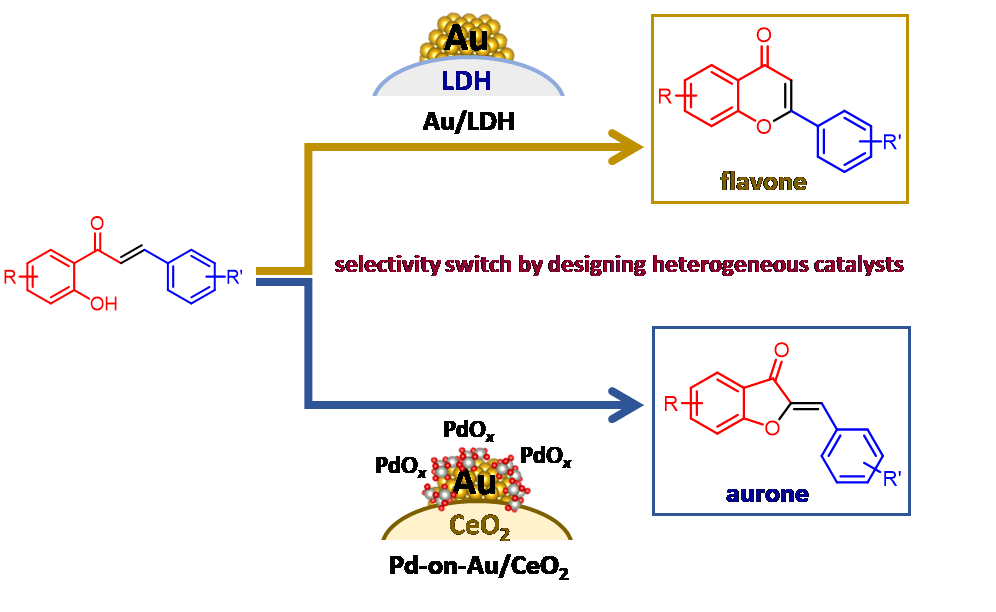
Furthermore, by designing a functionally integrated heterogeneous catalyst in which gold nanoparticles are supported on a basic hydroxide support LDH, a one-pot flavone synthesis from hydroxyacetophenones and aldehydes has been realized[1.9] (Fig. 1.3, top). Furthermore, it has been revealed that by designing a catalyst possessing a gold core-palladium oxide shell structure, aurones, which are very rare flavonoids, could be synthesized with high selectivity from chalcones for the first time[1.10] (Fig. 1.3, bottom). Such selectivity switches are unique to our precisely designed heterogeneous catalysts.
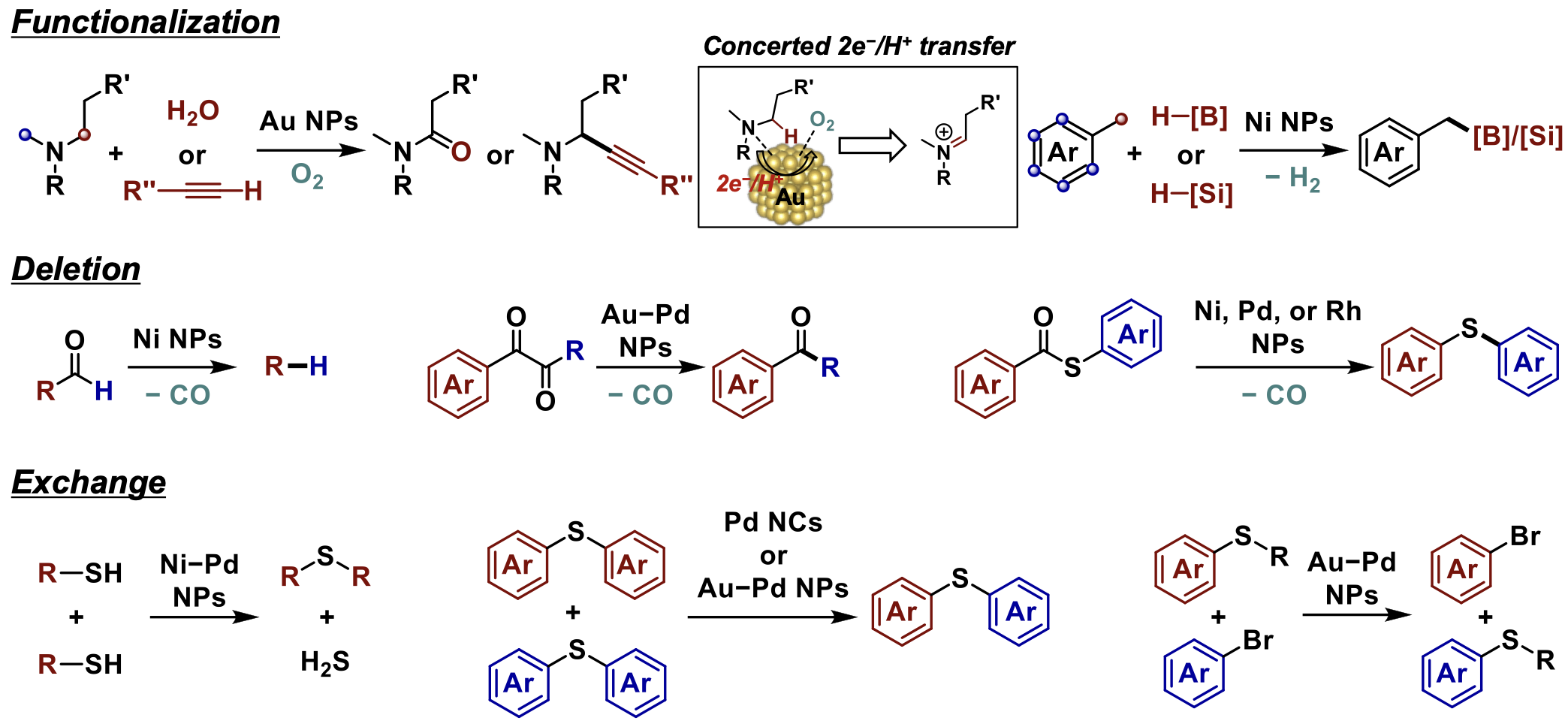
Recently, we have been also developing methods for efficiently synthesizing functional compounds by partially editing compounds possessing ubiquitous/useful structures. In fact, we have realized various molecular editing reactions, such as “functionalization” of specific C–H bonds, “deletion” of specific functional groups, and “exchange” of specific functional groups, by utilizing various metal nanoparticle catalysts (Fig. 1.4). For example, we have achieved α-methylene regiospecific oxidative functionalization of tertiary amines[1.11] (Fig. 1.4, top), regioselective dehydrogenative borylation/silylation at benzylic C(sp3)–H bonds of alkyl arenes[1.12] (Fig. 1.4, top), decarbonylation of carbonyl compounds like aldehydes, 1,2-diketones, and thioesters[1.13] (Fig. 1.4, middle), and C–S/S–H, C–S/C–S, and C–S/C–Br metathesis which involve the cleavage of C(sp2)–S bonds in sulfur-containing compounds such as sulfides and thiols[1.14] (Fig. 1.4, bottom). Many of these reactions are believed to be driven by unique multi-site activation on metal nanoparticle catalysts. For instance, in the aerobic oxidation of tertiary amines using gold nanoparticle catalysts, unprecedented concerted two-electron/one-proton transfer after co-adsorption of tertiary amines and molecular oxygen on gold nanoparticles is suggested as a new amine oxidation mechanism, leading to the previously unexplored α-methylene regiospecificity (Figure 1.4, top).
The above-mentioned reactions are unique to heterogeneous catalysts that have been realized for the first time by our precisely designed nanostructured catalysts. These reactions possess the following advantages; inexpensive and readily available raw materials, molecular oxygen as the oxidant, and/or ammonia as the nitrogen source can be used, and by-products are only water and/or molecular hydrogen. So far, investigations of heterogeneous catalysts have been dominated by simply immobilizing homogeneous catalysts for existing reactions and discussing their activities and reusabilities. On the other hand, our research is focused on creating and integrating active species unique to heterogeneous catalysts for developing new reactions that have not been realized even with well-designed homogeneous catalysts.
References
- K. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi, N. Mizuno, Science 2003, 300, 964-966.
- T. Oishi, K. Yamaguchi, N. Mizuno, Angew. Chem. Int. Ed. 2009, 48, 6286-6288.
- (a) K. Yamaguchi, H. Kobayashi, T. Oishi, N. Mizuno, Angew. Chem. Int. Ed. 2012, 51, 544-547; (b) Y. Wang, K. Yamaguchi, N. Mizuno, Angew. Chem. Int. Ed. 2012, 51, 7250-7253; (c) Y. Wang, H. Kobayashi, K. Yamaguchi, N. Mizuno, Chem. Commun. 2012, 48, 2642-2644; (d) Y. Miyamoto, Y. Kuroda, T. Uematsu, H. Oshikawa, N. Shibata, Y. Ikuhara, K. Suzuki, M. Hibino, K. Yamaguchi, N. Mizuno, Sci. Rep. 2015, 5, 15011.
- (a) X. Jin, K. Taniguchi, K. Yamaguchi, N. Mizuno, Chem. Sci. 2016, 7, 5371-5383; (b) X. Jin, K. Taniguchi, K. Yamaguchi, K. Nozaki, N. Mizuno, Chem. Commun. 2017, 53, 5267-5270.
- (a) K. Taniguchi, X. Jin, K. Yamaguchi, K. Nozaki, N. Mizuno, Chem. Sci. 2017, 8, 2131-2142; (b) Y. Koizumi, K. Taniguchi, X. Jin, K. Yamaguchi, K. Nozaki, N. Mizuno, Chem. Commun. 2017, 53, 10827-10830; (c) Y. Koizumi, X. Jin, T. Yatabe, R. Miyazaki, J. Hasegawa, K. Nozaki, N. Mizuno, K. Yamaguchi, Angew. Chem. Int. Ed. 2019, 58, 10893-10897; (d) S. Takayama, T. Yatabe, Y. Koizumi, X. Jin, K. Nozaki, N. Mizuno, K. Yamaguchi, Chem. Sci. 2020, 11, 4074-4084; (e) W.-C. Lin, T. Yatabe, K. Yamaguchi, Chem. Commun. 2021, 57, 6530-6533; (f) H. Li, T. Yatabe, S. Takayama, K. Yamaguchi, JACS Au 2023, 3, 1376-1384; (g) W.-C. Lin, T. Yatabe, K. Yamaguchi, Chem. Lett. 2025, 54, upaf043
- X. Jin, Y. Koizumi, K. Yamaguchi, K. Nozaki, N. Mizuno, J. Am. Chem. Soc. 2017, 139, 13821-13829.
- W.-C. Lin, T. Yatabe, H. Kimura, T. Yabe, K. Yamaguchi, ACS Catal. 2025, 15, 10651-10662.
- T. Matsuyama, T. Yatabe, T. Yabe, K. Yamaguchi, Nat. Commun. 2025, 16, 1118.
- T. Yatabe, X. Jin, K. Yamaguchi, N. Mizuno, Angew. Chem. Int. Ed. 2015, 54, 13302-13306.
- T. Yatabe, X. Jin, N. Mizuno, K. Yamaguchi, ACS Catal. 2018, 8, 4969-4978.
- (a) X. Jin, K. Kataoka, T. Yatabe, K. Yamaguchi, N. Mizuno, Angew. Chem. Int. Ed. 2016, 55, 7212-7217; (b) T. Yatabe, K. Yamaguchi, Nat. Commun. 2022, 13, 6505.
- (a) D. Yoshii, T. Yatabe, T. Yabe, K. Yamaguchi, ACS Catal. 2021, 11, 2150-2155; (b) Q. Yu, T. Yatabe, T. Matsuyama, T. Yabe, K. Yamaguchi, Catal. Sci. Technol. 2024, 14, 2730-2738.
- (a) T. Matsuyama, T. Yatabe, T. Yabe, K. Yamaguchi, ACS Catal. 2021, 11, 13745-13751; (b) T. Matsuyama, T. Yatabe, T. Yabe, K. Yamaguchi, ACS Catal. 2022, 12, 13600-13608; (c) T. Matsuyama, T. Yatabe, K. Yamaguchi, Org. Biomol. Chem. 2024, 22, 579-584; (d) T. Matsuyama, T. Yatabe, K. Yamaguchi, ACS Catal. 2024, 14, 10214-10222.
- (a) K. Mitamura, T. Yatabe, K. Yamamoto, T. Yabe, K. Suzuki, K. Yamaguchi, Chem. Commun. 2021, 57, 3749-3752; (b) T. Matsuyama, T. Yatabe, T. Yabe, K. Yamaguchi, Catal. Sci. Technol. 2024, 14, 76-82; (c) T. Matsuyama, T. Yatabe, T. Yabe, K. Yamaguchi, Chem. Sci. 2024, 15, 11884-11889; (d) T. Matsuyama, T. Yatabe, K. Yamaguchi, Cell Rep. Phys. Sci. 2025, 6, 102671.
Research 2 Conversion of natural carbon resource
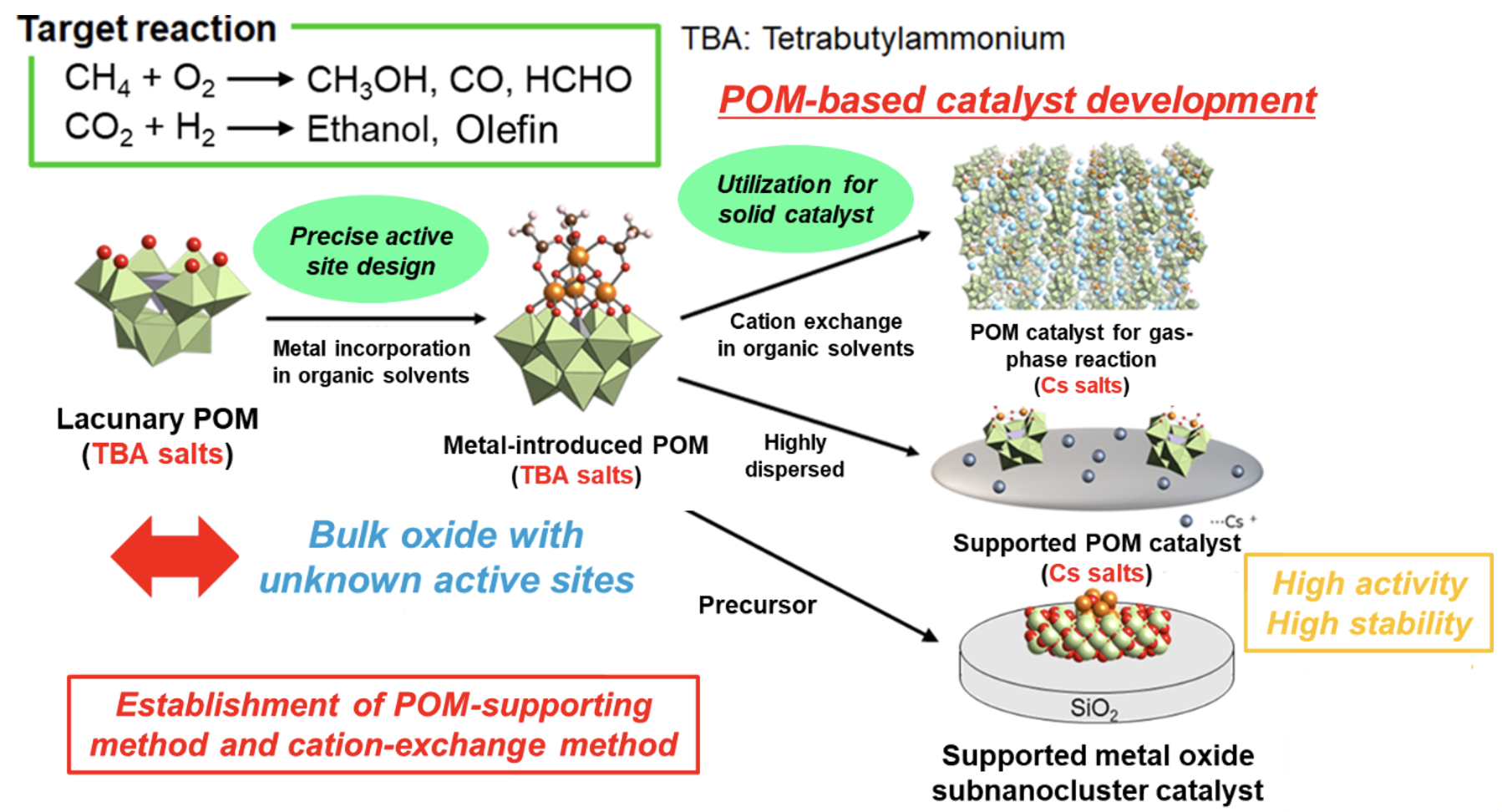
If we can efficiently obtain key chemical products from natural carbon resources such as methane—the main component of natural gas—and biomass resources, which are one of the unused carbon resources, it will contribute to the establishment of a future industrial infrastructure that utilizes diverse natural carbon resources in a balanced manner. We focus on the chemical conversion (especially oxidation reactions) of abundant natural carbon resources using catalysts. For example, in direct methanol synthesis[1] and direct formaldehyde synthesis[2,3] using methane, methanol and formaldehyde can be synthesized in a single step using oxygen, without passing through synthesis gas (a mixture of carbon monoxide and hydrogen). However, since the products are more reactive than the raw methane and easily undergo sequential oxidation, achieving high-yield and highly selective methanol synthesis is extremely challenging. In indirect conversion processes via synthesis gas, large-scale plants requiring multiple heat exchangers lead to high costs, so there is a demand for green chemistry-oriented, energy-efficient processes that enable direct on-demand production. Against this backdrop, catalyst development becomes a crucial factor. Polyoxometalates (POMs) are molecular inorganic oxide clusters with unique properties such as high redox capabilities and functions as inorganic molecular templates, depending on their constituent elements. We have been developing novel catalysts that enable the efficient conversion of carbon resources—including natural gas and biomass resources[4]—into key chemical products, utilizing POMs as precursors.
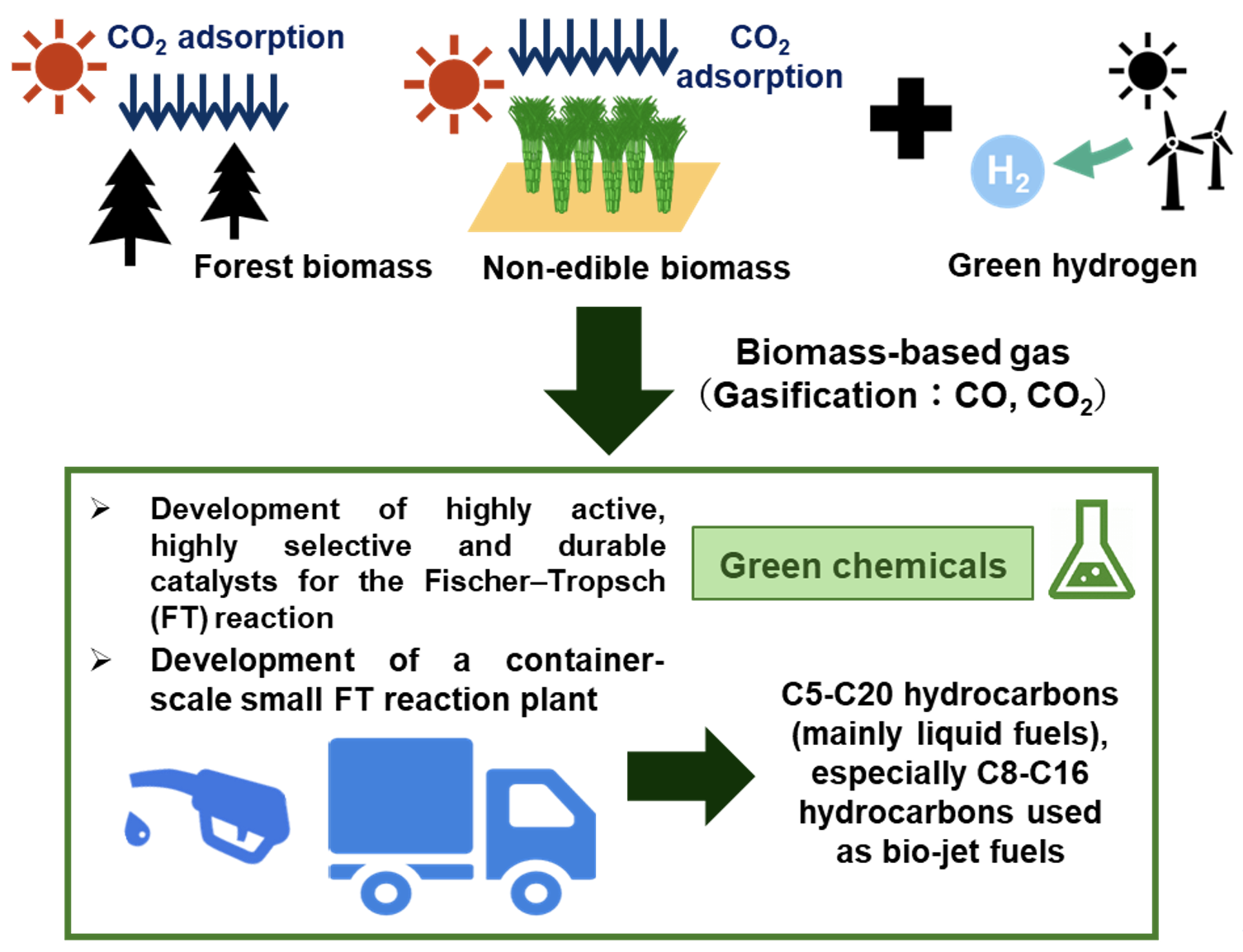
Recently, through the Fukushima Institute for Research, Education and Innovation (F-REI)[5] project, we have been conducting research and development on an integrated process to obtain valuable green chemical products. This process utilizes feedstock gas derived from Fukushima biomass (CO2, CO) as a carbon-neutral raw material and hydrogen sourced from renewable energy. We are focusing on catalyst development for hydrocarbon (fuel) synthesis via the highly efficient Fischer–Tropsch (FT) reaction. In conventional FT reactions, various hydrocarbons are synthesized according to the Anderson-Schulz-Flory (ASF) distribution, which dictates the carbon distribution of the products. To achieve selective synthesis of hydrocarbons that can serve as sustainable aviation fuel (SAF), we are developing novel catalysts using various porous materials and composite oxides as precursors to break away from the ASF distribution. Additionally, to accelerate catalyst development, we possess numerous advanced evaluation systems, including a single microreactor for rapid catalyst assessment and high-pressure gas flow reaction apparatuses capable of operating at pressures of up to approximately 4 MPa. Traditionally, catalyst development has often relied on existing catalysts combined with engineering approaches such as membrane separation and structured catalysts. However, we distinguish itself by employing a multifaceted approach integrating cutting-edge characterization techniques—such as atomic-resolution TEM—along with computational chemistry and high-throughput high-pressure reaction systems to develop highly active novel catalysts.
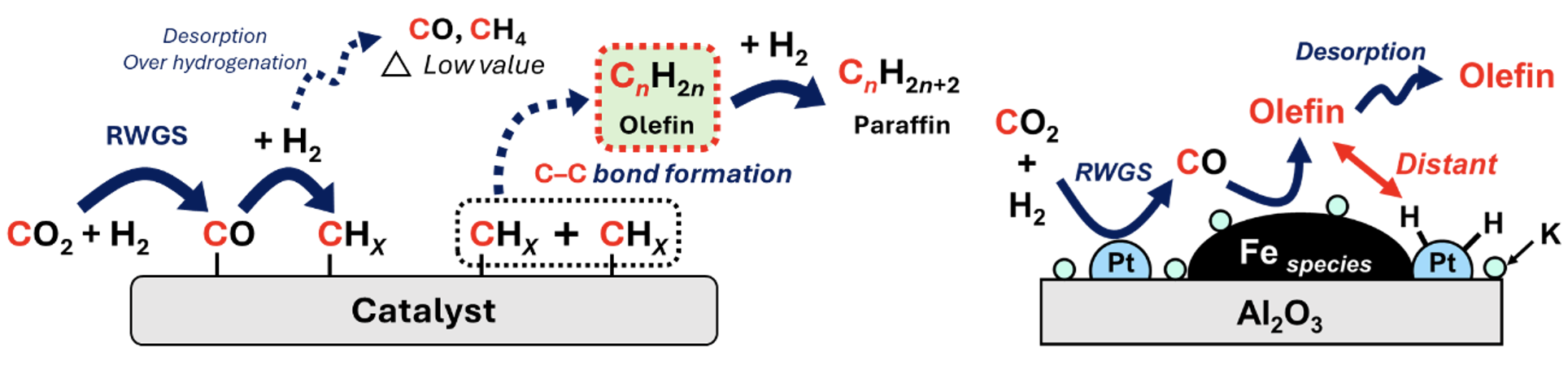
We are also developing catalysts for the hydrogenation of CO2 to synthesize lower hydrocarbons. As catalysts for synthesizing lower hydrocarbons—particularly lower olefins—from CO2, we have focused on iron-based catalysts utilizing iron carbide, which exhibits high activity in C–C bond formation. The CO2 hydrogenation reaction is kinetically limited by the preceding reverse water-gas shift (RWGS) reaction, requiring the addition of precious metals under low-pressure conditions. However, when precious metals such as ruthenium are added, undesirable methanation reactions occur as side reactions, leading to methane formation. To address this, we have successfully developed a catalyst in which ruthenium nanoparticles are supported on iron oxide. During the CO2 hydrogenation reaction, the iron oxide encapsulates the ruthenium, effectively suppressing the methanation reaction. To improve the yield of lower olefins, we are designing and utilizing active site arrangements and interfacial structures composed of iron and heterogeneous metals. This approach allows us to control hydrogenation ability and intermediate adsorption behavior, enabling the development of functionally integrated iron-based catalysts[6].
References
- T. Suzuki, T. Yabe, K. Suzuki, K. Yamaguchi, Chem. Lett. 2025, 54, upaf077.
- K. Wachi, T. Yabe, T. Suzuki, K. Yonesato, K. Suzuki, K. Yamaguchi, Appl. Catal. B 2022, 314, 121420.
- K. Wachi, T. Yabe, T. Suzuki, K. Yonesato, K. Suzuki, K. Yamaguchi, Catal. Sci. Technol. 2023, 13, 4744–4752.
- T. Suzuki, T. Yabe, K. Yamaguchi, J. Jpn. Petrol. Inst. 2025, in press.
- https://www.f-rei.go.jp/
- (a) T. Hojo, T. Yabe, K. Yamaguchi, J. Jpn. Petrol. Inst. 2023, 66, 238–245; (b) T. Hojo, T. Yabe, K. Yamaguchi, J. CO2 Util. 2025, 98, 103153.


