Photoreceptors (Microbial rhodopsins)
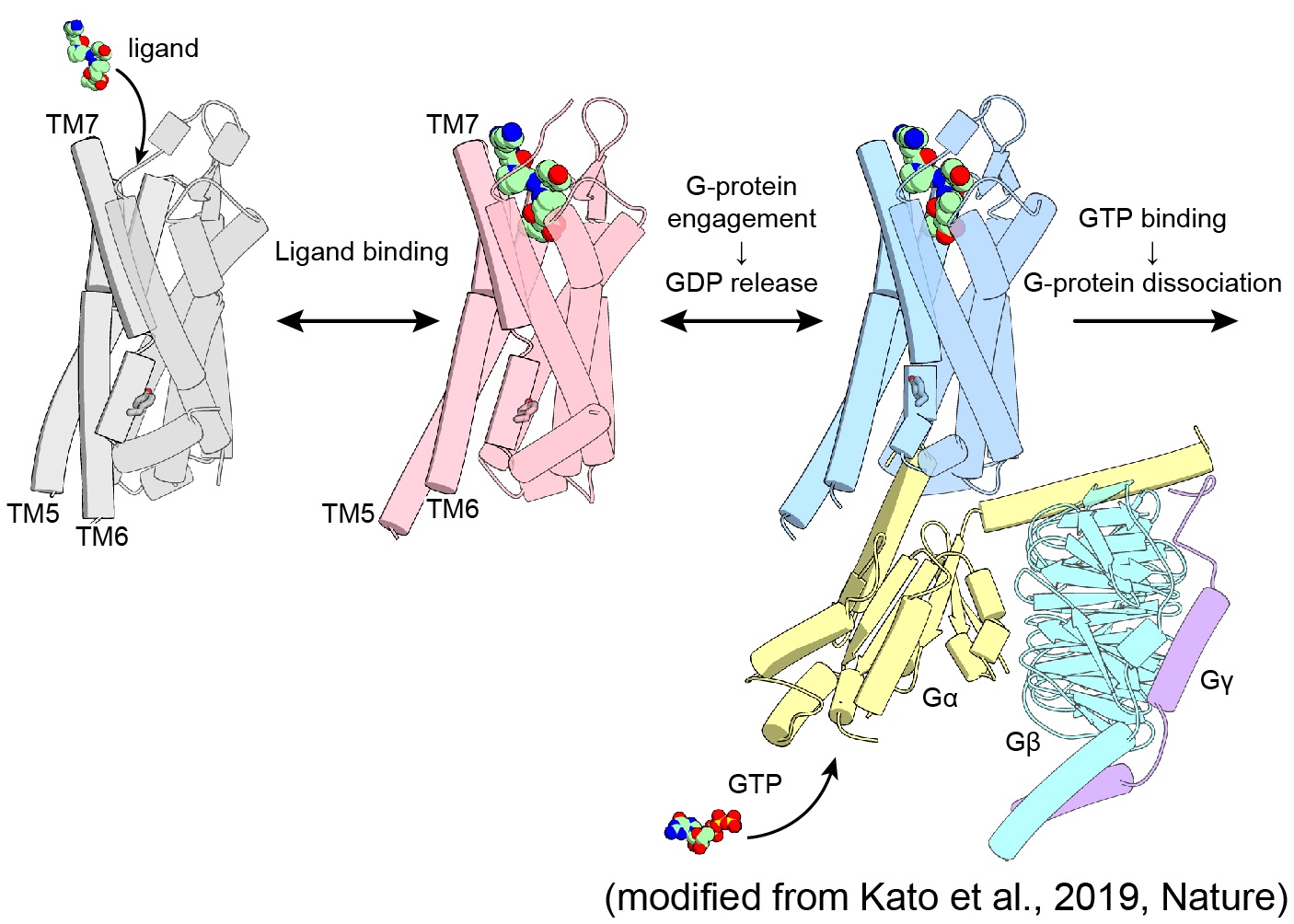
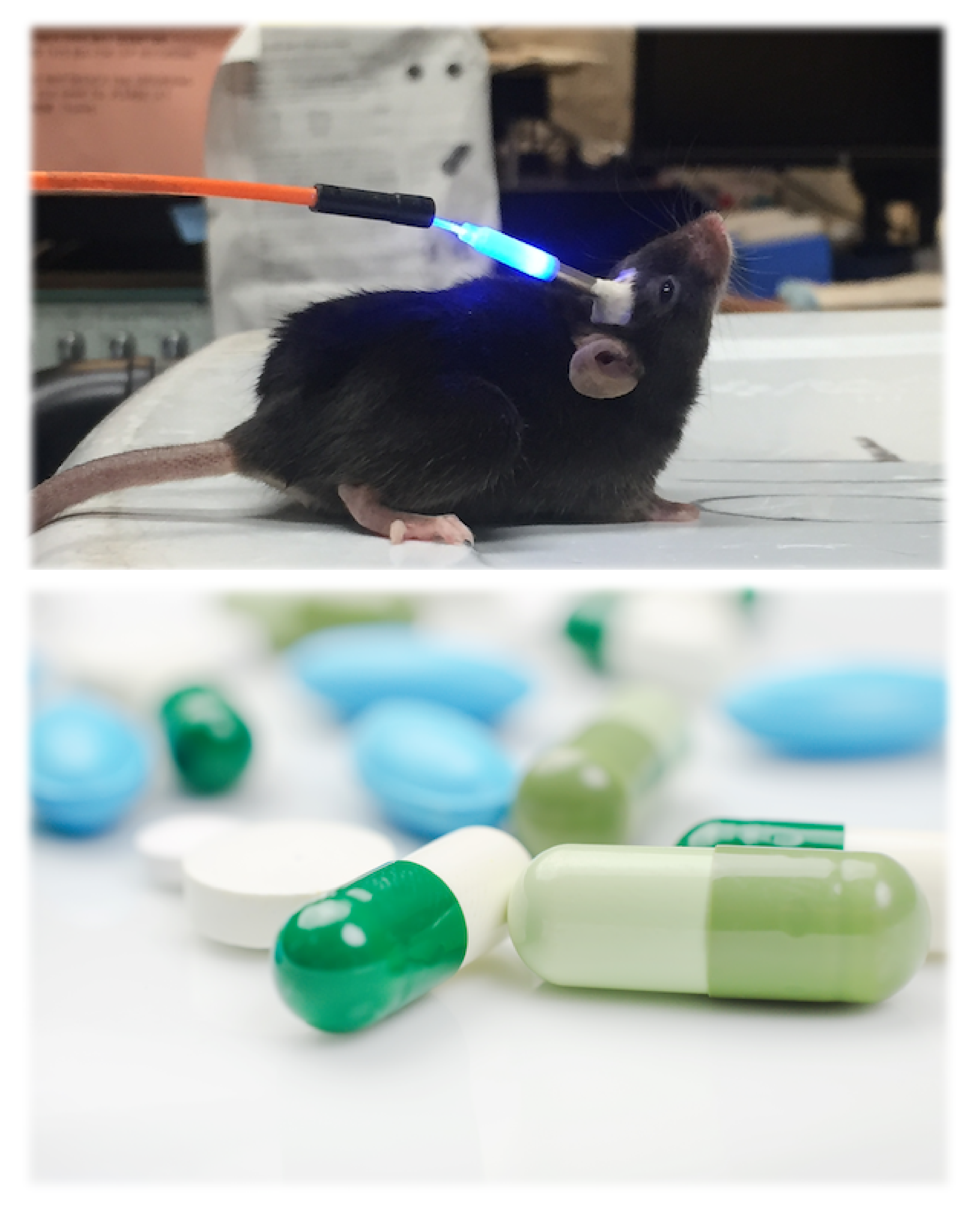
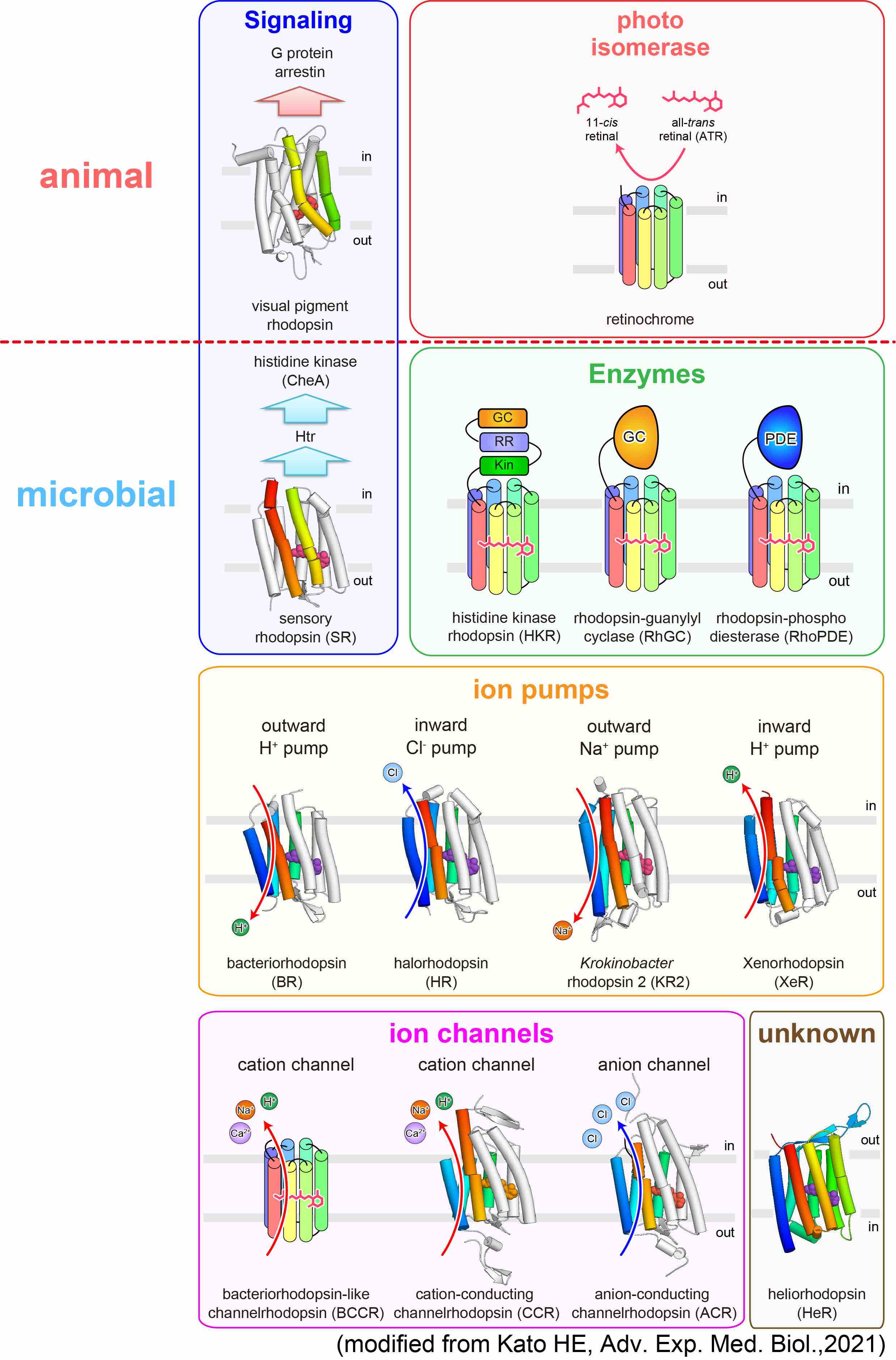
Most animals, from simple, single-celled microbes to large mammals like humasn, receive light information through rhodopsin family proteins. Rhodopsins form a large superfamily of seven-transmembrane proteins, categorized into type I (microbial) and type II (animal) based on their diverse sequences and functions. Recently, ion channel and ion pump rhodopsins have attracted broad attentions because of their remarkable utility to be used as a genetic toolkit to modulate neuronal activity with light (=optogenetics). We are studying these microbial rhodopsins using structural, biophysical, biochemical, and electrophysiological techniques to better understand their structure-function relationship.