When a substance functions as a material, interfacial interactions with other substances play a significant role. Although the ratio of the interface to the whole material is very small, most of the material’s properties and functions are determined by the interface. Our target is to design and synthesize new molecules that approach those interfaces, and develop new functional materials and synthetic methodologies. We are tackling this target by taking advantage of our strong background in organic and polymer chemisty from a viewpoint of both basic science and applications. Let's have fun with taking the very first step into the unexplored frontiers of materials science together!
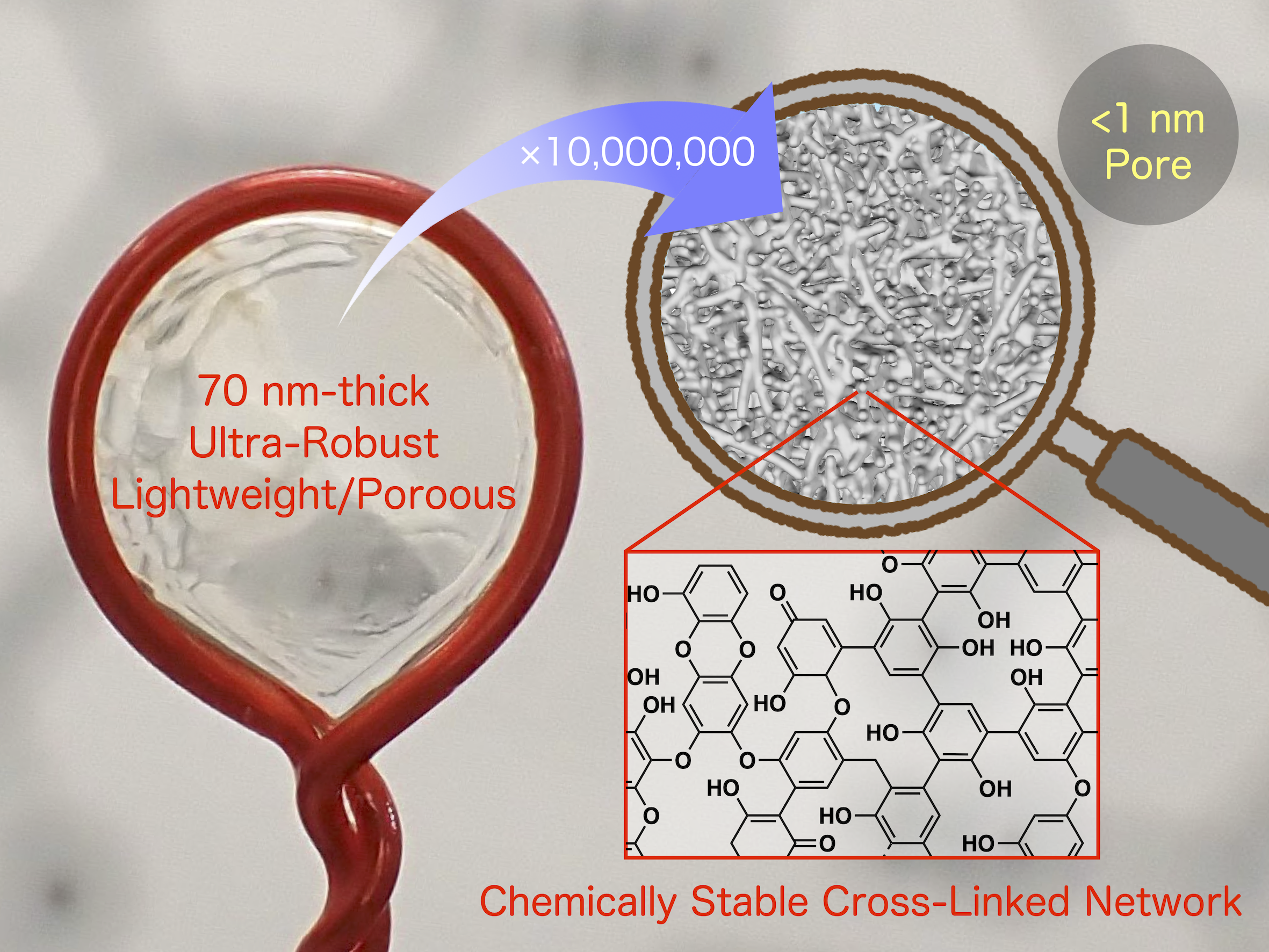
Porous, yet incredibly hard! We have developed a porous polymer that combines lightweight properties with exceptional strength, similar to a luffa sponge. Conventional porous polymers have been either brittle or soft. However, our novel polymer thin film defies expectations: it is 3-4 times harder than a typical polymer, despite having only half the density. This unique membrane is created using our unique and ingeniously simple "Columbus's egg" electrochemical method, which uses only water as a solvent. The resulting material is a mechanically robust and highly stable cross-linked polymer. With pore sizes just under one nanometer, it holds great promise for revolutionizing energy efficiency in difficult separation processes, including seawater desalination and various gas and oil separations.
Please contact Itoh for more information.
Science 2025, 389, 73-77. [DOI: 10.1126/science.adq0782]
Click here for free download.
Coming Soon!
How thin can we make a film with electropolymerization? We report a method for synthesizing infinitely thin film materials enabled by supramolecular interactions between electrodes and monomers.
Chemrxiv 2026. (preprint) [DOI:10.26434/chemrxiv.15000051/v1]
Coming Soon!
From the proteins in our bodies to high-performance polymers like aramid and polyamides, amides are everywhere in our life. They are known for their ability to link molecular parts together with hydrogen bonds. But what if they could do more? Our lab is unlocking the hidden potential of the amide's double bond character to pioneer revolutionary materials. Our targets are next-generation optoelectronic materials and sustainable, resource-saving polymers. Get ready for Amide 3.0—a new era for amides, where they do more than just hold molecules together!
Chemrxiv 2025. (preprint) [DOI:10.26434/chemrxiv-2025-cxfzs]
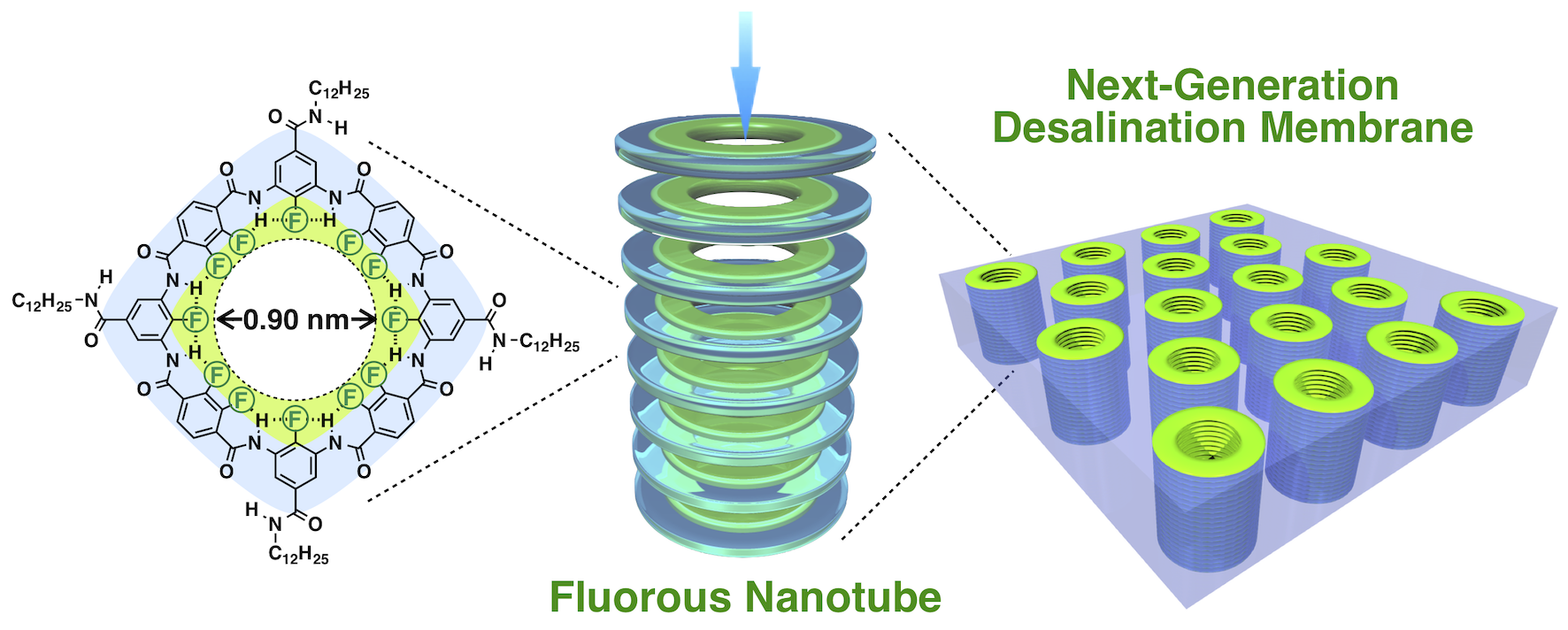
We discovered that tubes with an inner diameter of ~1 nm, whose inner surface is covered with fluorine atoms just like Teflon, allow water to pass through at an unprecedented rate but do not allow salt to pass through. It is expected to be applied to next-generation water purification membranes that surpass the performance of conventional desalination membranes.
Science 2022, 376, 738–743. [DOI: 10.1126/science.abd0966]
Others LINK
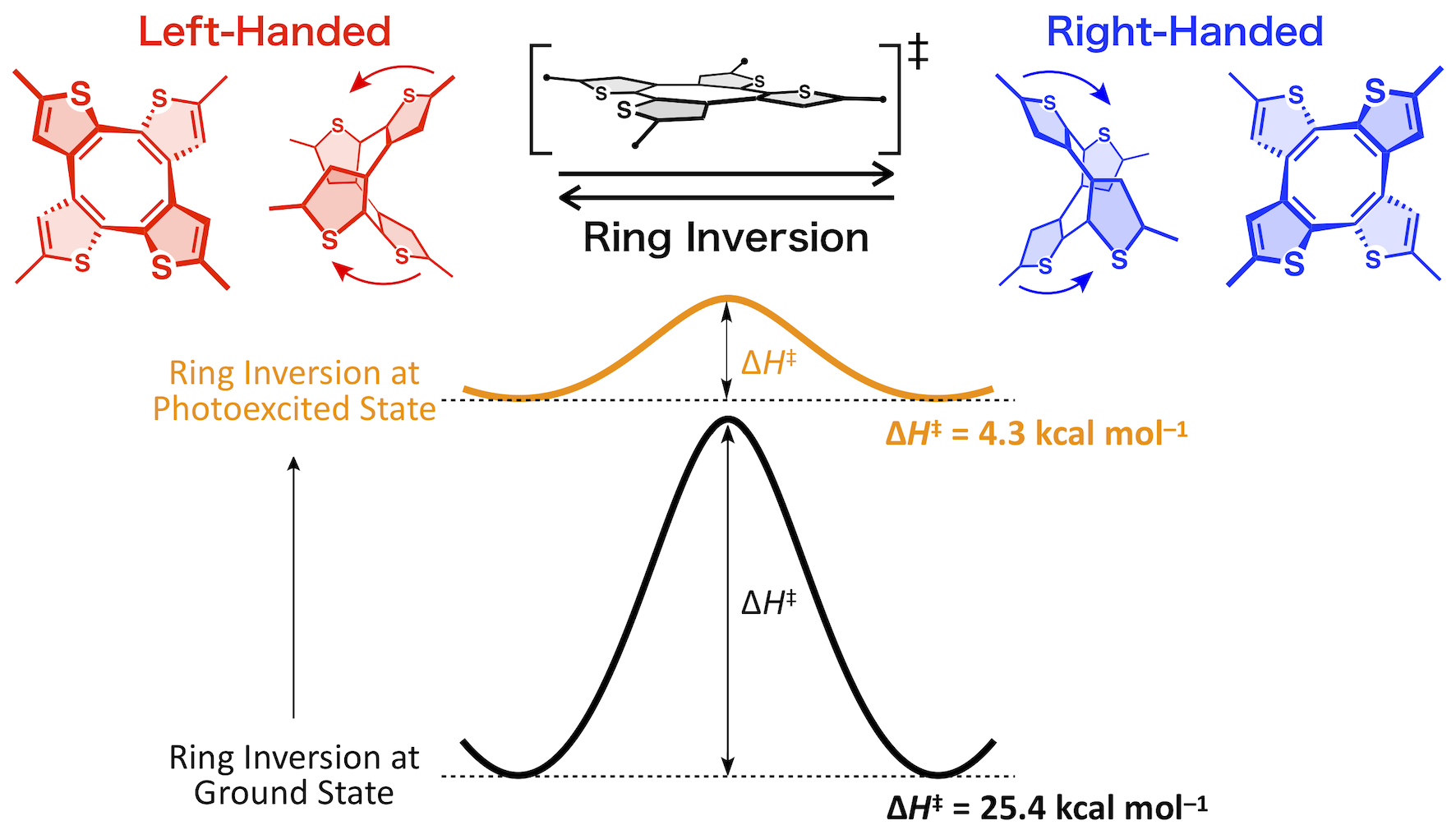
The stability of the cyclic π-electron conjugated skeleton depends on the number of π-electrons, which is summarized as Hückel’s rule. That is, when the skeleton has 4n+2 π-electrons, it is stabilized by "aromaticity," and when it has 4n π-electrons, it is destabilized by "anti-aromaticity." On the other hand, it is known that "aromaticity" also emerges in the photoexcited states, and this is summarized as Baird’s rule. This is the opposite of Hückel’s rule, which states that a molecule becomes aromatic when it has 4n π-electrons and anti-aromatic when it has 4n+2 π-electrons. Aromaticity contributes significantly to the stabilization of the molecule. Although the stabilization effect in the ground state has been investigated by various methods, it is not easy to investigate the stabilization effect in the photoexcited state. This is due to their extremely short lifetime of picoseconds to microseconds. We have experimentally revealed this effect for the first time by comparing the activation energies of the ring inversion barrier of thiophene-fused cyclooctatetraene in the ground state and in the photoexcited state. Using this molecular motif, we developed the world's first heterochiral supramolecular polymers and polymerization reactions that can be suspended by light.
Substantiation of the stabilization effect of the excited state aromaticity.
Nature Commun. 2017, 8, 346. [DOI: 10.1038/s41467-017-00382-1]
Chem-Station
The first alternating heterochiral supramolecular polymer.
J. Am. Chem. Soc. 2021, 143, 5121–5126. [DOI: 10.1021/jacs.1c00823]
The first photo-suspendable supramolecular polymerization.
J. Am. Chem. Soc. 2022, 144, 7080–7084. [DOI: 10.1021/jacs.2c02176]
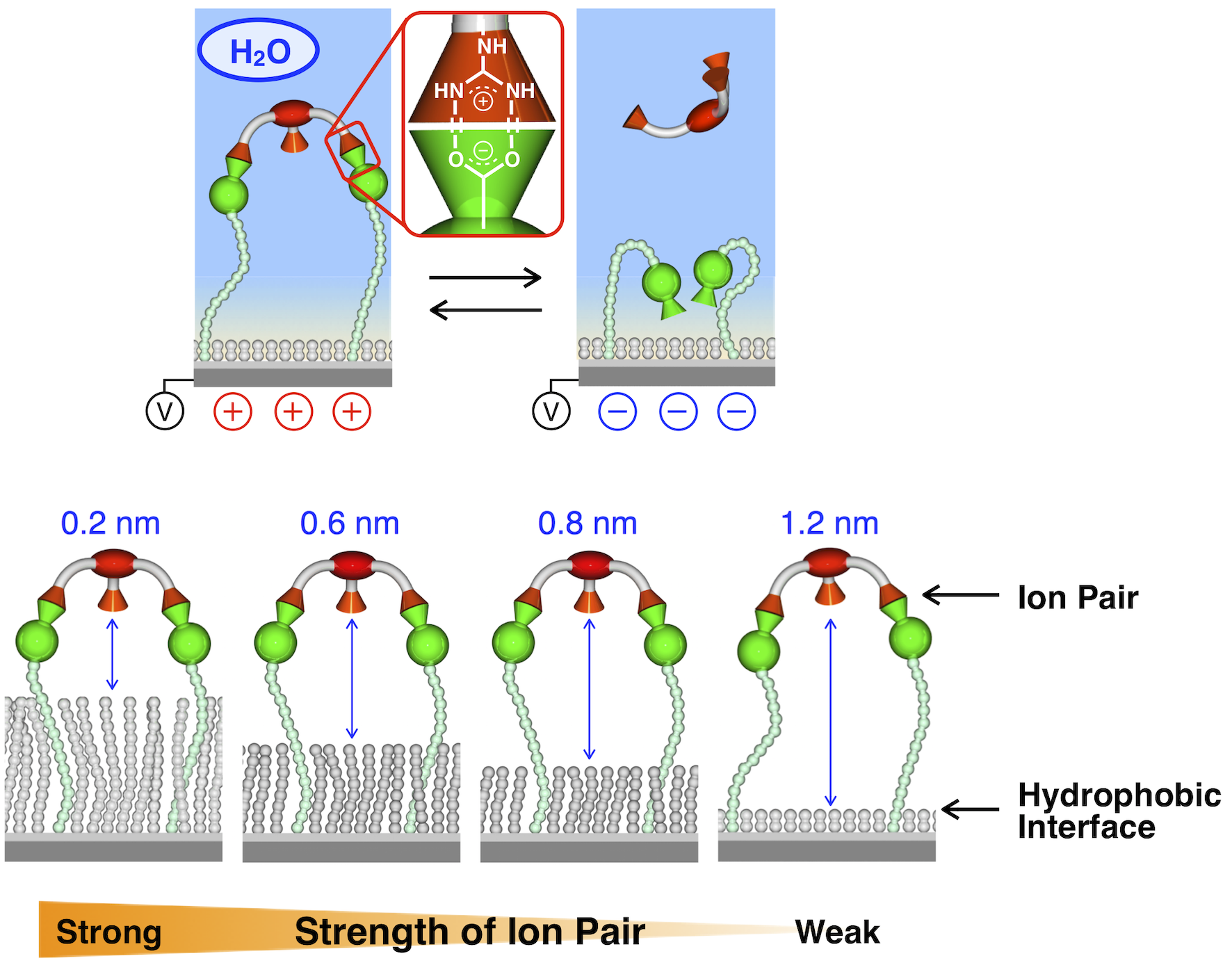
On biomolecular surfaces such as proteins, interactions with various molecules occur dynamically and are controlled by stimuli from the external field. We have successfully formed self-assembled monolayers (SAMs) on electrode surfaces and controlled their ionic bonds to the guest molecules in solution by electrode potential. During the course of the research project, we found that the shorter the distance between the ionic bonds and the hydrophobic alkyl group introduced to prevent exposure of the electrode surface, the stronger the ionic bonding is. This was the first substantiation of the theoretical prediction published in 1953 by Shellman. Later, we found an anomalous feature of molecular motion on hydrophobic interface. We also continue exploring the peculiar properties on electrode surfaces to develop interesting materials.
Development of E-field responsive SAMs and discovery of hydrophobic enhancement of ionic interaction.
Science 2015, 348, 555–559. [DOI: 10.1126/science.aaa7532]
Anomalous molecular dynamics on hydrophobic interface.
Chem. Asian. J. 2020, 15, 3321–3325. [DOI: 10.1002/asia.202000742]
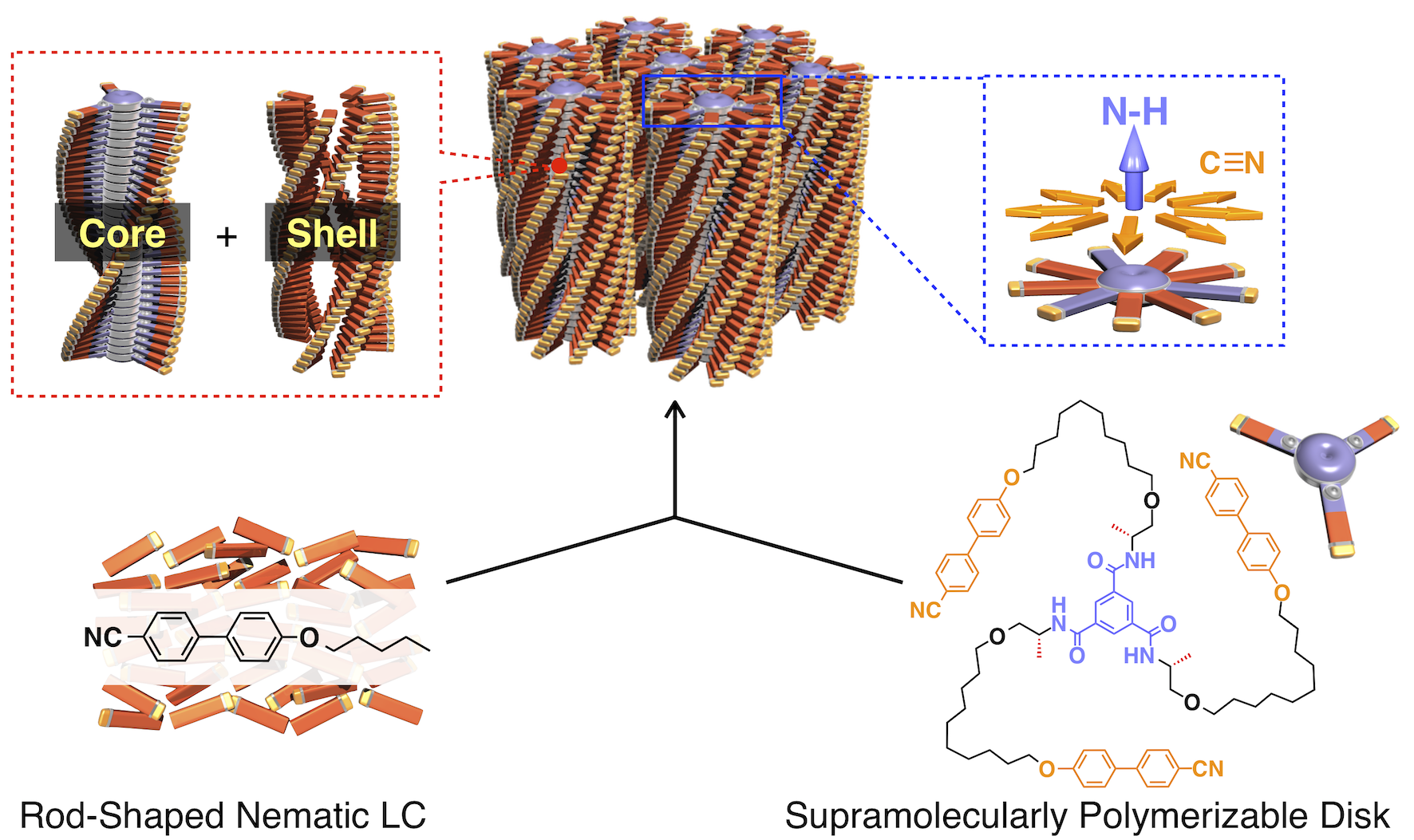
Rod-shaped molecules want to align parallel to each other, and disk-shaped molecules want to overlap each other face to face. Therefore, they generally do not mix without a solvent. By designing and synthesizing a new molecule, where a rod-like skeleton is introduced at the termini of the disk-shaped molecule, we have succeeded in obtaining a hybrid columnar liquid crystal in which rod- and disk-shaped molecules are mixed at the molecular level. This liquid crystal is the first columnar liquid crystal exhibiting dual-frequency response; the columnar axis orients parallel to DC electric field and perpendicular to AC electric field. Furthermore, by using photoresponsive molecules, we succeeded in obtaining for the first time a rewritable liquid crystalline AND logic gate that responds to both electric field and light. We were also able to design a columnar liquid crystal that responds to both electric field and magnetic field. We also found that the chirality of helical supramolecular polymers formed by disk-shaped molecules induces helical alignment of rod-shaped molecules. Conversely, chiral rod-shaped molecules induce an unusual chiral amplification phenomenon for achiral supramolecular polymers.
Hybrid columnar liquid crystal and AND logic gate.
Science 2019, 363, 161–165. [DOI: 10.1126/science.aan1019]
Chem-Station
Hybrid columnar liquid crystal responsive to both electric field and magnetic field.
J. Am. Chem. Soc. 2019, 141, 10033–10038. [DOI: 10.1021/jacs.9b03961]
Anomalous chiral transfer from chiral rod-shaped molecule to helical supramolecular polymer.
Chem. Asian. J. 2022, e202200223. [DOI: 10.1002/asia.202200223]
Supramolecular copolymerization of hydrophilic and hydrophobic monomers in liquid crystalline media.
Chem. Sci. 2024. 15, 4068-4074. [DOI: 10.1039/d3sc06341k]
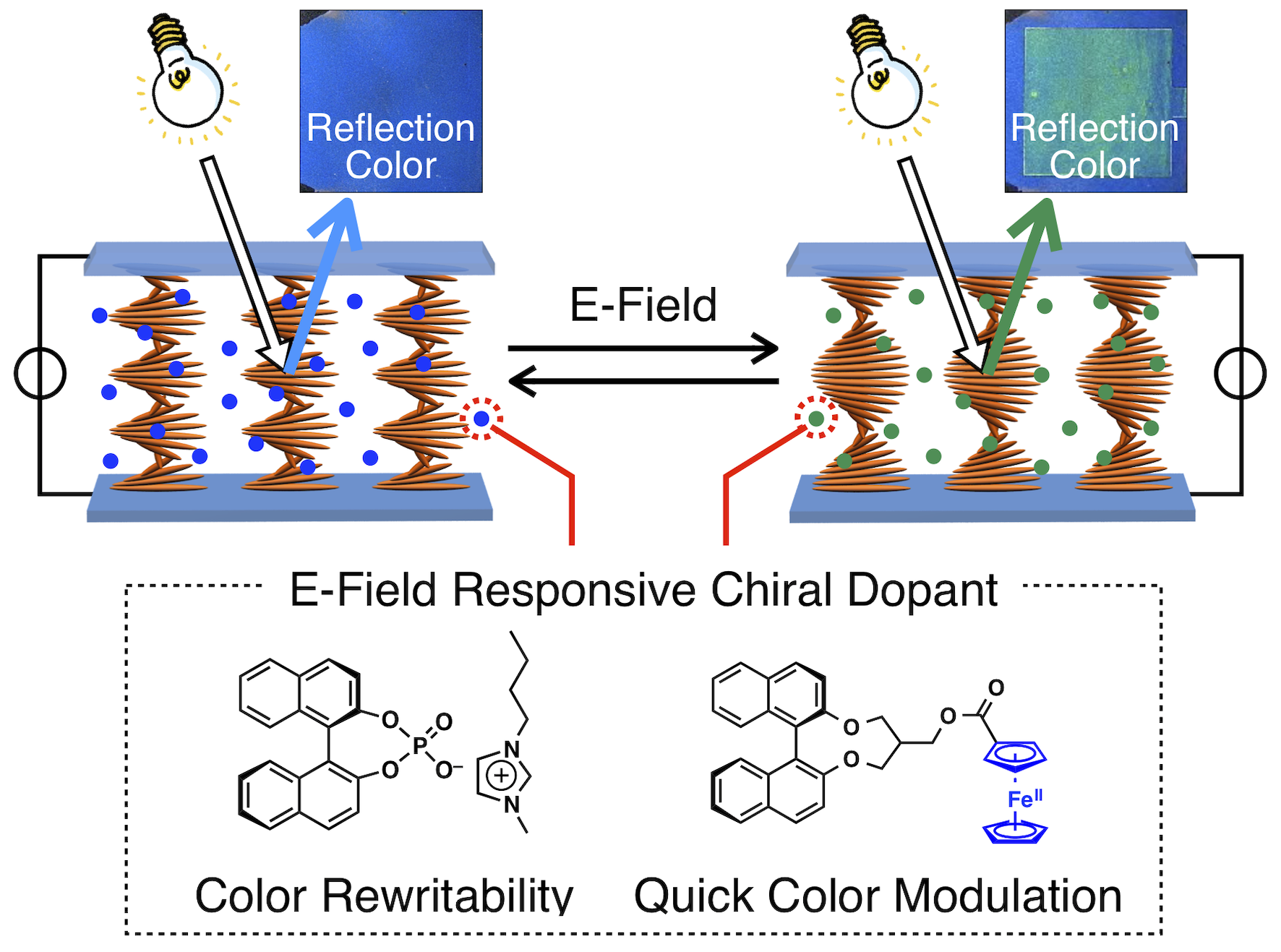
Cholesteric liquid crystals have an internal helical structure and can selectively reflect light of wavelengths corresponding to their pitch (structural color). We have succeeded in changing the structural color of cholesteric liquid crystals by using an E-field, and have realized two new reflective displays. One is a device that can write and rewrite reflection colors, and the other is a display device that can be driven at high speed by a single dry cell battery (1.5 V) using a redox reaction. The key is the molecular design that gives ionic properties to the chiral dopants that create the helical structure. Conventionally, ionic compounds have been thought to degrade the quality of liquid crystalline devices. However, through the deep consideration of the chemical principles, we have shown that ions in liquid crystals can be used as functional materials. In the device for the first study, they were time-consuming but they had a color memory function. In the device for the second study, they can be driven at high speed but the color cannot be memorized. If this "best of both worlds" can be achieved, it will lead to full-color electronic paper.
Cholesteric display device whose color can be written and rewritable.
Adv. Mater. 2016, 28, 4077–4083. [DOI: 10.1002/adma.201600258]
Cholesteric display device whose reflection color can be changed quickly.
J. Am. Chem. Soc. 2018, 140, 10946–10949. [DOI: 10.1021/jacs.8b06323]
Device fabrication video
J. Vis. Exp. 2019, 144, e59244. [DOI: 10.3791/59244]