- Home
- Research
Research
Innovation of New Energy Resources and Foundation of New Social Systems
All across the world, people are facing energy problems on a global scale. As part of a paradigm shift on energy resources, development of new energy resources such as artificial photosynthesis has been investigated to escape from the present situation that mainly depends on fossil fuels. One practical way to solve this problem is to use renewable energy such as sunlight, wind power, tidal power, geothermal power and so forth.
However, conversion of energy into high energy materials leading to new energy resources widely available in society is necessary to use renewable energy efficiently.
Our laboratory is challenging the generation of new energy resources and an innovative social system using it.
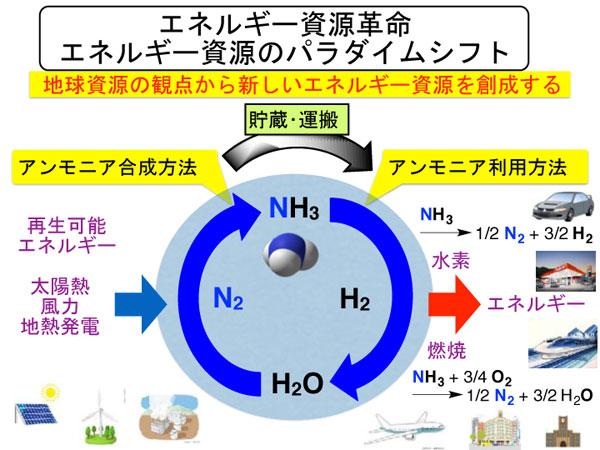
Development of New Catalysis Technology to Convert Ammonia into Resources
Ammonia has been synthesized from the atmospheric dinitrogen and is now expected as a new energy resource that emits only water and dinitrogen.
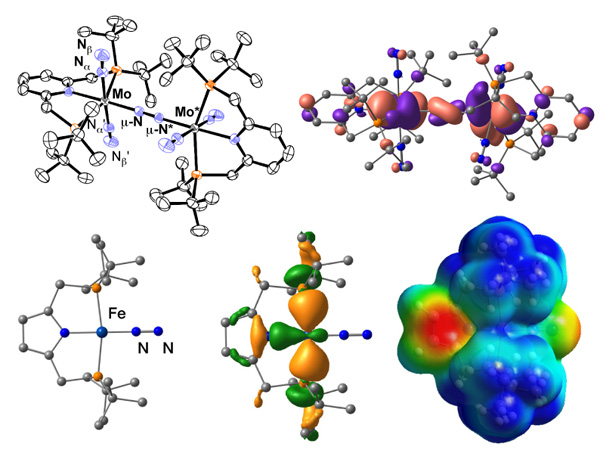
We are developing next-generation nitrogen fixation catalytic system necessary to realize "ammonia society" where ammonia is used as an energy resource. We have designed several dinitrogen complexes that can work as molecular catalysts to convert dinitrogen into ammonia under mild reaction conditions.
Development of Novel Catalyses as Solutions to Shortage of Energy Resources
Plastic and chemical products such as pharmaceuticals around ours are synthesized by accumulation of chemical reactions using catalyst. The development of more efficient synthesis method is important from the viewpoint of resources and energy. Chemical products such as plastics and pharmaceuticals are synthesized by several chemical reactions. Thus, development of more efficient synthetic methods is important to solve the shortage of energy resources. Our group is working on the development of novel and practical chemical reactions for the future.
Keywords
Energy Chemistry, Molecular Catalysis, Ammonia, Chemical Reaction, Chemical Synthesis
This laboratory is working on experimental chemistry where new molecules are synthesized by members of this laboratory, aiming to nurture talented persons with flexible thinking to lead the future. Please come and join our research team!
Cover Picture
 Synthesis and Redox Properties of PNP Pincer Complexes Based on N-Methyl-4,4'-Bipyridinium
Synthesis and Redox Properties of PNP Pincer Complexes Based on N-Methyl-4,4'-BipyridiniumY. Miyake, K. Nakajima, Y. Higuchi, and Y. Nishibayashi
Eur. J. Inorg. Chem., 4273-4280 (2014).
[Highlighted at Cover Picture]
 Molybdenum-Catalyzed Reduction of Molecular Dinitrogen under Mild Reaction Conditions
Molybdenum-Catalyzed Reduction of Molecular Dinitrogen under Mild Reaction ConditionsY. Nishibayashi
Dalton Transactions (Perspective, invitation), 41, 7447-7453 (2012).
[Highlighted at Cover Picture]

Cooperative Catalytic Reactions Using Lewis Acid Catalysts and Organocatalysts: Enantioselective Propargylic Alkylation of Propargylic Alcohols Bearing Internal Alkyne with Aldehydes
K. Motoyama, M. Ikeda, Y. Miyake, and Y. Nishibayashi
Eur. J. Org. Chem., 2239-2246 (2011).
[Highlighted at Cover Picture]
 Cooperative Catalytic Reactions Using Organocatalysts and Transition Metal Catalysts: Enantioselective Propargylic Alkylation of Propargylic Alcohols with Aldehydes
Cooperative Catalytic Reactions Using Organocatalysts and Transition Metal Catalysts: Enantioselective Propargylic Alkylation of Propargylic Alcohols with AldehydesM. Ikeda, Y. Miyake, and Y. Nishibayashi
Angew. Chem. Int. Ed., 49, 7289-7293 (2010).
[Highlighted at Cover Picture]
 Enantioselective Ring-Opening Reactions of Racemic Ethynyl Epoxides via Copper-Allenylidene Intermediates: Efficient Approach to Chiral b-Amino Alcohols
Enantioselective Ring-Opening Reactions of Racemic Ethynyl Epoxides via Copper-Allenylidene Intermediates: Efficient Approach to Chiral b-Amino AlcoholsG. Hattori, A. Yoshida, Y. Miyake, and Y. Nishibayashi
J. Org. Chem., 74, 7603-7607 (2009).
[Highlighted at Cover Picture]
 Ruthenium-Catalyzed Enantioselective Propargylation of Aromatic Compounds with Propargylic Alcohols via Allenylidene Intermediates
Ruthenium-Catalyzed Enantioselective Propargylation of Aromatic Compounds with Propargylic Alcohols via Allenylidene IntermediatesH. Matsuzawa, Y. Miyake, and Y. Nishibayashi
Angew. Chem. Int. Ed., 46, 6488-6491 (2007).
[Highlighted at Cover Picture]

