RESEARCH
During the 21st century, humans will face serious problems regarding environmental pollution and food shortages. To solve these problems, it will be important to apply the useful biological functions of plants and microorganisms more efficiently. To achieve this goal, we have to understand the molecular bases of regulatory mechanisms for physiological phenomena in higher plants and novel metabolic capacities of useful microorganisms, and to utilize the fruits of basic research for actual application.
Our research subjects are largely divided into two parts:(1) MICROBIAL RESEARCH: Analysis of novel metabolic capacities of bacteria for xenobiotics and its application for bioremediation of environmental pollution and (2) PLANT RESEARCH: Elucidation of regulatory mechanisms of disease resistance in higher plants and its application for the development of non-toxic agrochemicals to induce disease resistance and rice cultivars resistant to pathogens and for production of useful substances
Microbial Research
Under updating. Please wait for a while. Coming soon
<>PLANT RESEARCH
The research subjects are directed by Associate Prof. Okada.
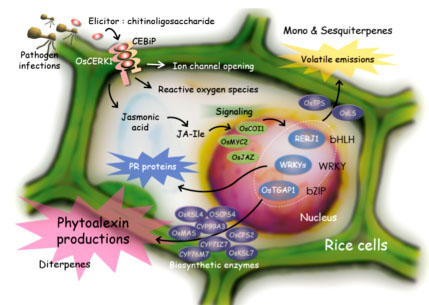
Higher plants recognize pathogen attack when pathogen-derived elicitors, such as chitin and β-glucan oligomers, bind to specific cell surface receptors. The elicitor perception leads to defense responses, such as cell death, production of phytoalexins, and expression of pathogenesis-related (PR) proteins. Phytoalexins are plant antibiotics, that is, secondary metabolites with anti-microbial activity, and PR proteins also show anti-microbial activity. These defense responses are caused via a series of signal transduction pathways including production of secondary signaling molecules, such as jasmonic acid (JA) and reactive oxygen species (ROS), and expression and/or activation of transcription factors followed by activation of target genes (Figure 1-1).
To elucidate the signal transduction pathways leading to expression of defense responses in higher plants, we have performed extensive studies using rice and/or Arabidopsis as plant materials and obtained the followings results:
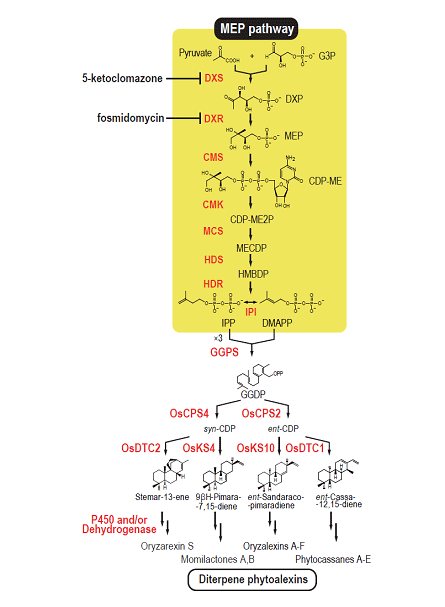
(a)We clarified the outline of biosynthetic pathways of rice diterpenoid phytoalexins (Figure 1-2),
and obtained important findings about regulatory mechanisms of the expression of those biosynthetic enzyme genes, such as identification of elicitor-responsive cis-elements of a biosynthetic gene followed by identification of a transcription factor involved in the gene expression, and identification of a biosynthetic gene cluster for the major phytoalexins momilactones.
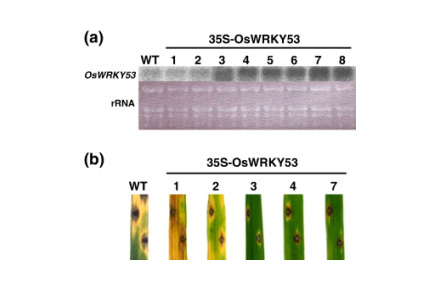
(b)We carried out detailed characterizations of the transcription factor genes OsWRKY53, OsWRKY71, and RERJ1 that are early responsive to a chitin oligosaccharide elicitor/ JA to strongly suggest that these transcription factors play important roles in defense responses in rice. In fact, overexpression of OsWRKY53 in rice plants enhanced disease resistance to a pathogen, Magnaporthe grisea (Figure 1-3),
Figure 1-3. Effects of overexpression of OsWRKY53 on resistance to M. grisea race 007 in rice plants. (a) Overexpression of OsWRKY53 in transgenic rice plants. Northern analysis was performed using total RNA (10µg) prepared from transgenic rice plants. Ethidium bromide staining of rRNA confirmed equal loading of total RNA in each lane. The numbers at the top of the gels indicate independent transgenic rice cells. VC, vector control. (b) Responses of leaf blades of 35S-OsWRKY53 transgenic rice plants to infection by the compatible M. grisea race 007. Lesions were observed 1 week after infection.
although the effect of overexpression of OsWRKY71 or RERJ1 on disease resistance in rice plants is now being examined. We further prepared rice cell lines transformed with a plasmid harboring a promoter region of OsWRKY53 or RERJ1 fused to a firefly luciferase reporter gene and an attempt to develop screening systems of agrochemicals enhancing disease resistance using those cell lines is also in progress.
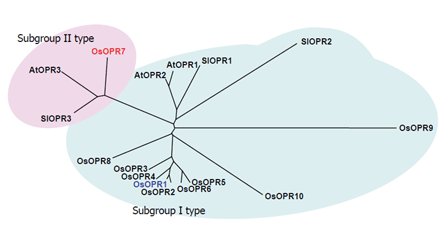
(c)By differential screening we isolated a cDNA of a gene, OsOPR1, as a JA-induced gene from suspension-cultured rice cells. Nucleotide sequence analysis indicated that the cDNA encodes a 12-oxophytodienoic acid (OPDA) reductase (OPR), which is involved in the biosynthesis of JA. We indicated that OsOPR1 convert (-)-cis-OPDA preferentially, rather than (+)-cis-OPDA, a natural precursor of JA, suggesting that OsOPR1 may have other biological functions in the reduction of unknown substrates other than OPDA. It was also shown that a bZIP transcription factor is involved in regulation of OsOPR1 gene expression. We further indicated that an OPR family gene in rice chromosome 8, designated OsOPR7, encodes the enzyme involved in the JA biosynthesis (Figure 1-4).
Figure 1-4. Phylogenetic relationship of OPRs between rice and other plants. The amino acid sequences deduced from OPR cDNA of rice (OsOPRs), tomato (SlOPRs), and Arabidopsis (AtOPRs) were compared using the program CLUSTALW. The phylogenetic tree was generated from the resulting alignment using the program TREEVIEW. Subgroup I and II type OPRs are indicated by shaded circles.
(d)We performed a characterization of a JA-deficient mutant cpm2, and suggested that cpm2 will be a useful tool to investigate the biological roles of JA.
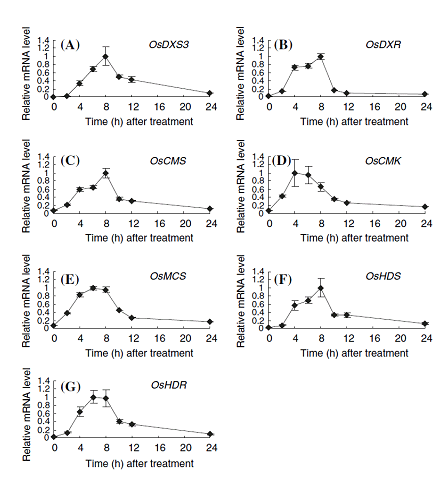
Terpenoids, the largest group of natural products in living organisms, are derived from a basic five-carbon unit, isopentenyl diphosphate (IPP), and its allyl isomer dimethylallyl diphosphate (DMAPP). IPP is sequentially condensed to DMAPP to yield the short-chain isoprenoid precursors geranyl diphosphate, farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP), which are further metabolized to monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20). IPP and DMAPP biosyntheses occur via two independent pathways in plant: the well-known mevalonate (MVA) pathway in cytoplasm and the methylerythritol phosphate (MEP) pathway in plastids. Above mentioned phytoalexins, secondary metabolites with anti-microbial activity, is one of diterpenes that are thought to be produced mainly via the MEP pathway.
(e)We found that all seven genes most likely responsible for the MEP pathway are coordinately expressed by the elicitor treatment accompanied by the expressions of the genes for phytoalexin biosynthesis. The MEP pathway is essential for the biosynthesis of terpenoid plant hormones including gibberellins, abscisic acids, and cytokinins. Therefore, the coordinate upregulation of the MEP pathway-gene expression by the elicitor treatment seems to be a major strategy of plants to supply sufficient terpenoid precursors for the production of phytoalexins in infected rice plants without a shortage of the important diterpene plant hormones (Figure 1-5).
Figure 1-5. Expression profiles of possible genes in the MEP pathway in cultured-cells. The total RNAs used for expression profiling were isolated from rice cells exposed to 1 ppm chitin elicitor for the indicated times. Values indicate relative mRNA levels normalized to the expression of the UBQ gene, and the maximal value in each experiment with different primers was arbitrarily set to 1.0. A, OsDXS3; B, OsDXR; C, OsCMS; D, OsCMK; E, OsMCS; F, OsHDS; G, OsHDR. The results are the average of at least three independent experiments; bars indicate the standard deviation of the mean.