Project 2. Artificial Peptide Drug Discovery
In living systems, biomolecules such as proteins and nucleic acids form complex structures and exert their functions by recognizing and interacting with each other. Understanding the principles behind molecular recognition is expected to enable the development of molecular tools for manipulating biological phenomena, ultimately leading to the discovery of new drugs. We are conducting research aimed at achieving peptide-based drug discovery.
Design of Cyclic Peptides with High Cell Membrane Permeability
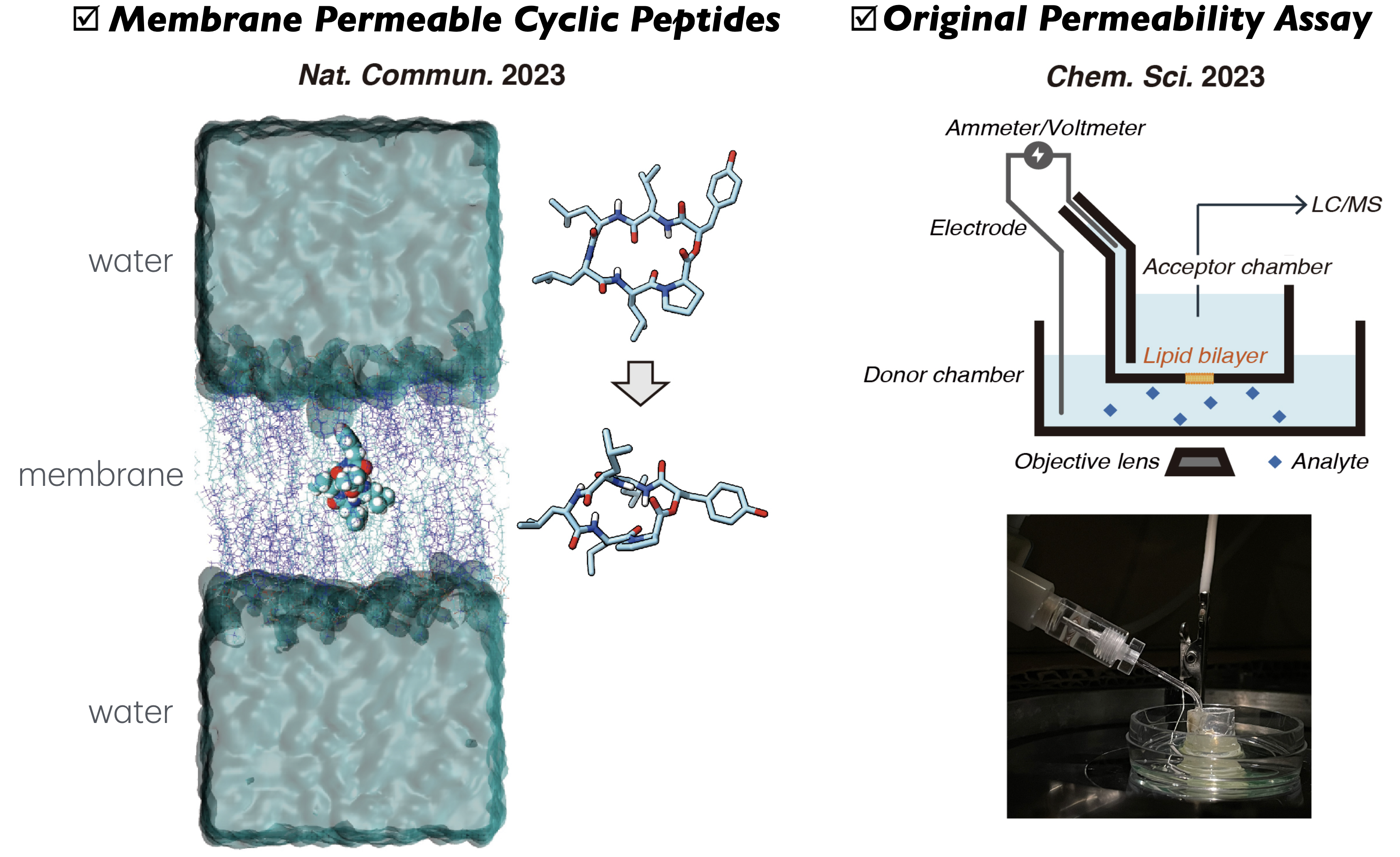
Cyclic peptides are attracting attention as next-generation drug modalities because they can adopt diverse three-dimensional structures, enabling precise molecular recognition. However, conventional peptides generally exhibit low passive cell membrane permeability, which remains a major limitation for their application in drug discovery. We are conducting research aimed at overcoming this critical challenge and achieving breakthroughs in peptide-based drug discovery
Based on our original membrane permeability evaluation methods and detailed structural analyses, we have elucidated the mechanisms by which cyclic peptides cross cell membranes passively. Utilizing these insights, we are designing cyclic peptides with high passive membrane permeability. Currently, we are taking on the challenge of creating functional peptides with both membrane permeability and biological activity through large-scale screening using DNA-encoded peptide libraries and data science approaches.
[References] Nature Commun. 2023, 14, 1416. Chem. Sci. 2023, 14, 345–349.
Peptoids. Biomolecular Recognition by Synthetic Oligomers
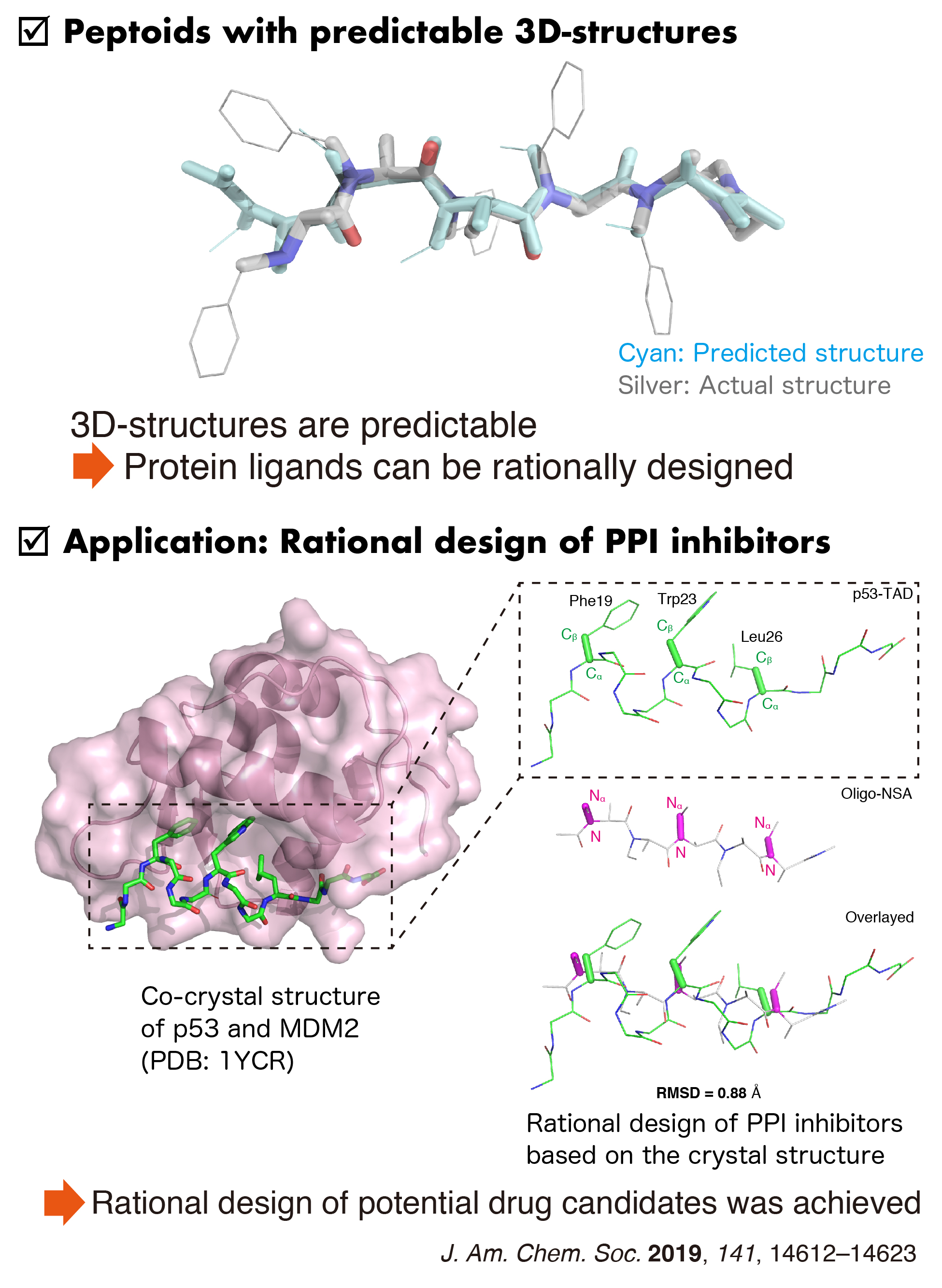
Synthetic oligomers called "peptoids" are known to be highly membrane-permeable peptidomimetics. The term "peptoids" indicates N-substituted peptides. Due to the lack of amide hydrogens, peptoids exhibit higher memrbane permeabilities than peptides. The regular peptoids are conformationally flexible due to the glycyl and β-alanyl backbones, which is potentially limiting the affinity of peptoids. Recently, by introducing chiral substituents on backbone carbons, we have succeeded in producing new peptoids that are conformationally constrained. We have demonstrated that the new peptoid, i.e., oligo(N-substituted alanines), can be utilized for rationally designing molecules that recognize protein surfaces.
[References] Chem Sci. 2025, 16, 10512–10522. Chem Sci. 2024, 15, 7051–7060. Angew. Chem., Int. Ed. 2022, 61, e202200119. Chem. Sci. 2021, 12, 13292–13300. J. Am. Chem. Soc. 2020, 142, 2277–2284. J. Am. Chem. Soc. 2019, 141, 14612–14623.
