The Univ. of Tokyo > Graduate School of Agricultural and Life Sciences > Department of Applied Biological Chemistry > Lab. of Radio-Plant Physiology
The Univ. of Tokyo > Graduate School of Agricultural and Life Sciences > Radioisotope Center > Lab. of Radio-Plant Physiology





Overview
Our group has been trying to make use of radioisotopes in order to investigate substance dynamics and distribution within the plant, which was not possible using conventional techniques. It is because we think that knowledge on the motion of substances essential to life is an important key for fully understanding life activities.
We have developed various devices such as a real-time imaging system for visualising substance dynamics with radionuclides or an analysing technique for very low amount samples. Moreover, we have been producing radionuclides with very short half-life, for example magnesium 28, on our own to use them in our experiments. This allowed us to analyse the motion of nutrients in the plant’s body in a non-destructive manner, which was not possible before. Furthermore, we succeeded in the imaging of the distribution of plant hormones, which was thought to be hardly achievable due to their very low amount.
After the Great East Japan Earthquake, we have also been analysing radionuclides motion in the environment, outside our laboratory, i.e. dynamics of radioactive caesium, which contaminated agricultural lands and forests, and we are transmitting the knowledge thus obtained to the citizens as swiftly as possible.
Our main research themes are the following:
- -Real time Radioisotope Imaging System
- -Analysis of absorption and transport of magnesium in plants
- -Improvement of oil production by Brassicaceae
- -Previous study themes
Real time Radioisotope Imaging System
Introduction
There are 17 elements needed for a plant’s growth. Plants grow by selectively absorbing and metabolizing these elements. For these reasons, we supplement the soil with these elements through fertilization, in order to improve crop’s growth. However, fertilization is often based on experience, and the whole picture of the mechanisms by which the plant controls its own absorption of minerals is still unclear. If we can study the relationship between the plant’s growth and its nutritional elements in detail, it is thought that more efficient fertilization and a better use of the plant’s growth potential can be achieved.
In plant’s physiology, nutritional elements were mainly studied for the investigation of the responses to the impairments created by their deficiency or excess. This past few years, analyses from molecular biology and membrane biophysics allowed the gradual understanding of molecular structures involved in each nutritional element’s acquisition and transport. Moreover, a few genes working during nutrient impairment have been identified, thanks to massive analysis if genetic expression. However, information on the most fundamental question when dealing with responses, “How do plants judge nutrient excess or deficiency?” is still the object of extensive research, and in order to clarify this, it is necessary to understand in detail how elements are transported or accumulated. In such context, we have been focusing on the nutritional elements themselves, and we have been analyzing the mechanisms of absorption by the plant and transport within its body.
Developing and Improving the Imaging System
Until the experimental use of radioisotopes, plant physiologists could not measure the flow of inorganic ions quantitatively and with high sensitivity inside plant tissues. Radioisotopes started to be used in the early 1950s, and helped obtaining new knowledge in inorganic nutrition of plants. A remarkable example is potassium, which is the most abundant cation in the plant cell: an experiment using an isotope of potassium (42K) and one of its homologue 86Rb as tracer showed that absorption and transport of potassium is dependent on the ionic concentration of the surrounding environment. Tracing inorganic ions labelled with radioisotope is still the only way to study their absorption, translocation and outflow, and remains to be a classical experimental method until now.
However, one of the problems when conducting these experiments is that it is a destructive method, involving dissection of the plant and fixation on the X-ray film or the Imaging Plate (Fuji film) for measuring the tracer. Moreover, it is not easy to analyze the temporal changes, even when tracing more than one identical individual. Nevertheless, considering that the situation inside the living plant is continuously changing, tracing ions while maintaining the plant’s physiological activity would be ideal. Our laboratory has been developing new equipment for achieving our goal: “Imaging the radioactive tracer inside the living plant”.
The detection of radioactive isotopes may be roughly divided into methods directly detecting the radiations, and methods detecting light converted from radiation by scintillation reaction. Our system is an application of the latter principle. Since light detection techniques improved to the extent of detection of a single photon, the use of a high-sensitivity camera for detecting weak light has been thought for high-sensitivity detection of radioactivity. The mechanism of the system is the following: the plant sample is put in contact to the plate-shaped scintillator (CsI-Tl), the radiations emitted from inside the plant are converted into visible light and detected by a MCP equipped camera (Figure 1).

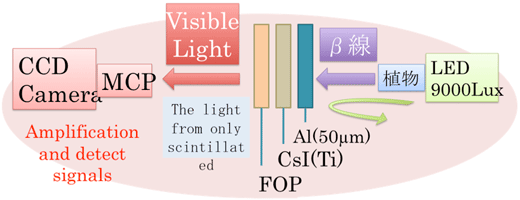
Fig. 1 Left: outside view of the imaging system Right: principle of the imaging system
When comparing this system with the versatile Imaging Plate, the sensitivity was 20 times higher with the same analytic capacity. For this reason, we succeeded in taking one picture in up to 2 minutes, and by linking continuously taken pictures, we could obtain a dynamic image 1) 2). Currently, it is possible to observe phosphorus (32P), sulfur (35S), calcium (45Ca), carbon (14C) and cadmium (109Cd) with this system.
Initially, we have been using 5cm×5cm scintillator plates to take photographs of each organs such as the leaf or the seed of the plant, but the increasing of the scintillator plate’s surface area and improvements on plant mounting allowed the observation of the whole plant at the same time. In addition, although the photograph needed to be taken in a black box, due to the high-sensitivity camera’s characteristic, we decided to keep the physical activity of the plant by placing a box inside the black box, so that light irradiates only on the mounted plant (Figure 2).
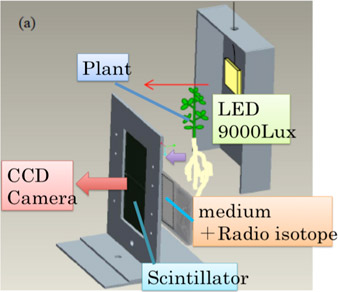
Fig. 2 Mechanism of light irradiation on the sample plant
Applications to plant physiology: analysis of the response of plants to low phosphorus environment
Our laboratory is conducting research on phosphorus, an essential macro-element amongst plant’s essential elements. The first thing seen when analyzing the imaging of phosphorus (32P) is that the transport of phosphorus within the plant is controlled by the age of each organ. If we take the example of soy leaves, it is clear that, between old and young leaves, there is predominant accumulation and transport of phosphorus to young growing leaves and shoots (Figure 3). Furthermore, its distribution was uneven between veins within a single leaf, so it has been thought that control of the transport within the plant was occurring at the cellular level.
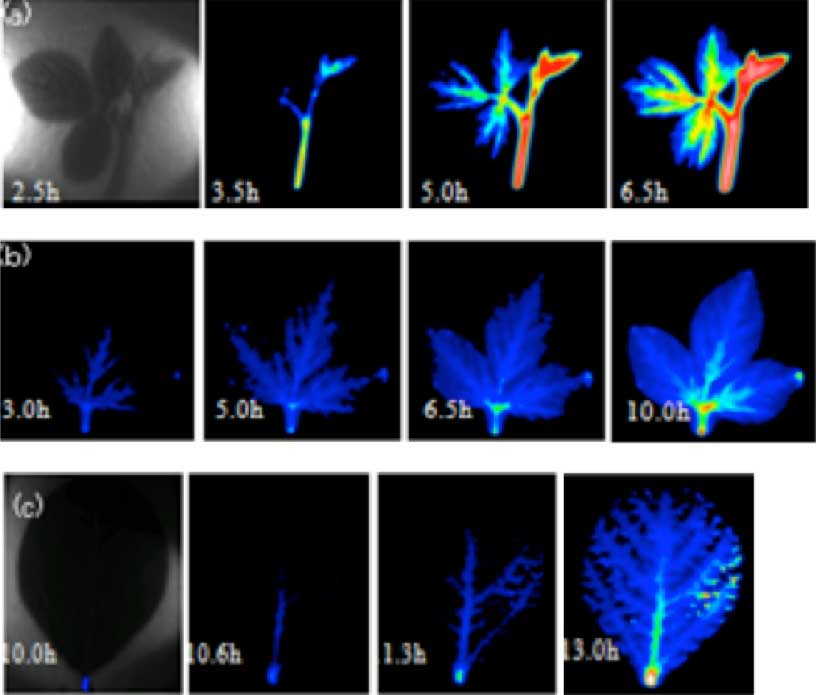
Analysis of 32P dynamics by radioisotope imaging by organs
Photographs taken after providing liquid-grown 35 days old soya roots (or seed stem) with 32P containing culture liquid (10MBq/50ml Hoagland). One picture was taken for 3 min in total. (a) young leaf and shoot; (b) grown-up leaf; (c) leaf on an even later growing stage
Following this experiment, we compared the differences in phosphorus transport between the growing stages (juvenile stage, budding stage, seed development stage) through imaging, since we are now able to observe the whole individual at once. During bud and seed development periods, it has been shown that phosphorus is massively transported to these tissues in comparison with other organs (Figure 4).
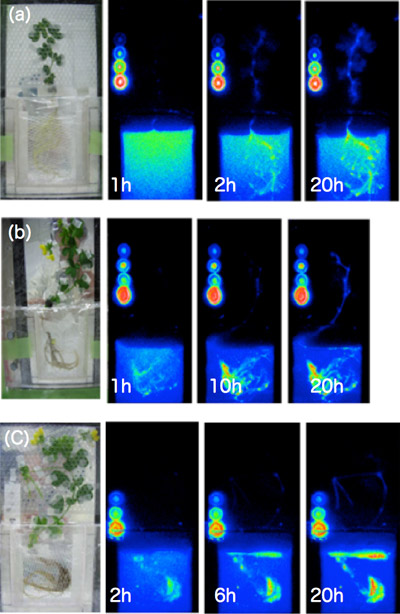
Fig. 4 Analysis of 32P dynamics by radioisotope imaging on the whole plant
Photographs taken after providing liquid-grown 20 to 45 days old Lotus japonicus with 32P containing culture liquid (1MBq/30ml Hoagland). One photograph has been taken for 3 min in total. (a) plant grown for 20 days, phosphorus spread evenly; (b) during budding stage, transport to the leaves was reduced compared to the juvenile stage; (c) during seed formation stage, phosphorus is promptly transported and accumulated to seeds. The circles in the picture represent 1, 5, 10 and 25 kBq of 32P respectively
It is known from previous studies that, in a phosphorus deficient environment, plants promote root development and improve their phosphorus taking in capacity by changing the number and type of proteins in root membranes that selectively take phosphorus in. However, there had not been any reports on transport of the actual transport substrate, phosphorus, so we tried to analyze by imaging the changes in transport when grown in limited phosphoric nutrient conditions. It appeared that when phosphoric nutrients were suspended in the middle of its growth period, the plant was absorbing and transporting to the stem and leaves 7 times as much phosphorus as in abundant conditions, and we could also calculate from the picture the transport velocity. We have thus been presenting imaging analysis as a new analyzing method of plant research.
From now on
The next interesting thing after the dynamic substance transport within the whole individual plant is the dynamics of the intercellular and intracellular inorganic ions’ dynamics. In our laboratory, we have been trying to apply the detection system mentioned above to the microscope for imaging. We are currently working to make refinements in order to improve its analytic capacity. In the future, we would like to apply this technique for simultaneous observation of fluorescently-labelled local proteins or expressed genetic loci and inorganic ions, to clarify the mechanisms by which the plant answers to inorganic nutrients.
Analysis of absorption and transport of magnesium in plants
Mg as a nutrient
For plants, magnesium is an essential macro element, and its ionic concentration within the plant cell is the second highest after potassium. Not only essential for maintaining the activity of aver 300 enzymes, Mg also plays an important role in forming RNA's higher order structure as well as osmoregulation of the cell. In plants, it is a central element in chlorophyll, thus essential for photosynthesis. We would like to understand how plants respond to Mg excess or deficiency to maintain development, through the analysis of the response mechanism to Mg deficiency, the Mg absorption/transport mechanism, and the Mg transport molecules.
Analyzing the mechanism of response to Mg deficiency
When a plant lacks Mg, the leaves turn yellow and over accumulation of starch occurs amongst other symptoms. Usually, these symptoms of Mg deficiency are seen when Mg is removed from the culture medium for more than a week. Moreover, a characteristic of Mg deficiency is that these symptoms are prominently seen in young leaves in growth period.
In our laboratory, we are trying to find out the process which leads to the onset of Mg deficiency using 2 weeks old rice plants. In rice, the first young leaf (L5) which grew during Mg shortage treatment (0 mM Mg) was the most sensitive to Mg deficiency. On the 8th day after the beginning of Mg shortage, starch over accumulation and reduction in chlorophyll content was observed; when re-supplying Mg after this date, the leaf did not recover and finally died. However, the leaf which grew right after this one (L6), although it showed starch over accumulation and chlorophyll content reduction at the same period, did recover after Mg re-supply. This means that Mg deficiency described above cannot be considered as a factor determining the leaf's life or death.
Therefore, we analyzed the temporal changes of physiological activity in each leaf, and we revealed that the factor determining the rice leaf's death is the reduction in the transpiration activity. This reduction in transpiration activity could be seen in L5 6 days after the beginning of Mg shortage, but was not in L6 even after the 8th day. It can thus be thought that L6, which showed an over accumulation of starch and reduction in chlorophyll content, could recover when re-supplied with Mg because transpiration was maintained and the Mg re-supplied through the roots translocated.
In order to clarify the mechanisms by which the transpiration activity is reduced, we are currently studying the changes at the molecular and genetic levels occurring a few days after the beginning of Mg shortage.
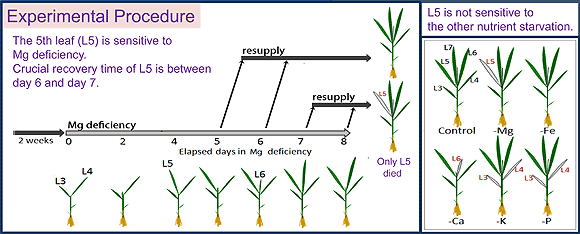
Fig. 1
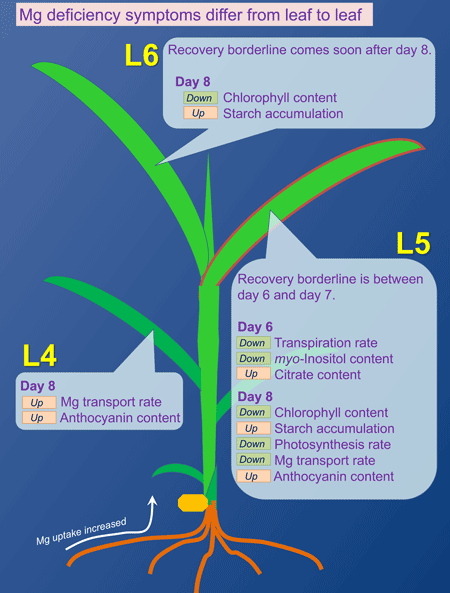
Fig. 2





