Research @ UTokyo
This page introduces some of the research being conducted in our laboratory.
 |
 |
 |
 |
 |
 |
 |
 |
1. Gas phase cluster studies toward multi-element catalysts replacing Pt and Rh
Platinum and rhodium are essential materials in automotive exhaust gas purification catalysts because of their high catalytic activity. However, they are rare metals with high costs and finite amounts of resources. Therefore, catalysts made of inexpensive and abundant elements instead of rare metals are actively investigated. As one of the approaches, we are trying to design a catalyst using multi-element materials. In order to avoid getting stuck in the complicated problem of "which elements" and "in what proportions", we use an original method to analyze the reactivity efficiently using the gas-phase cluster technique.
In particular, we have recently been working with Toyota Motor Corporation to develop new catalysts for decomposition of NO and CO without using rare elements such as Pt and Rh.
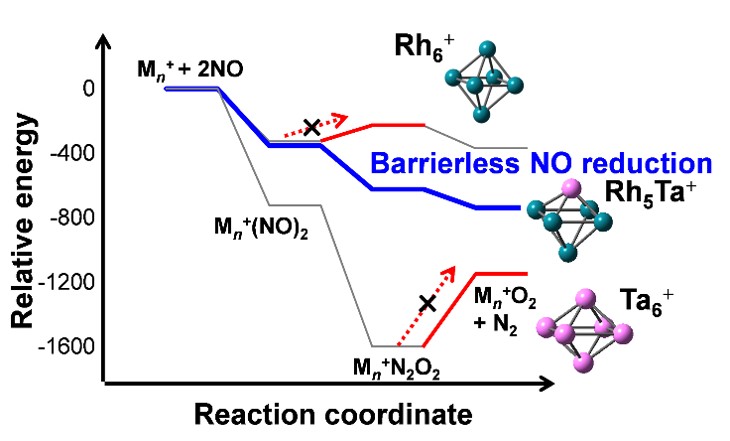 Reactions of Rh-Ta clusters with NO molecules |
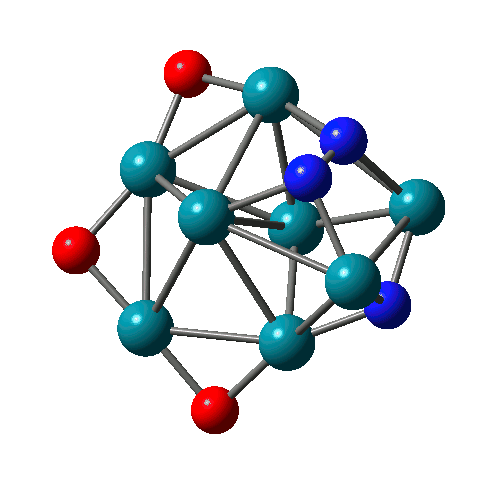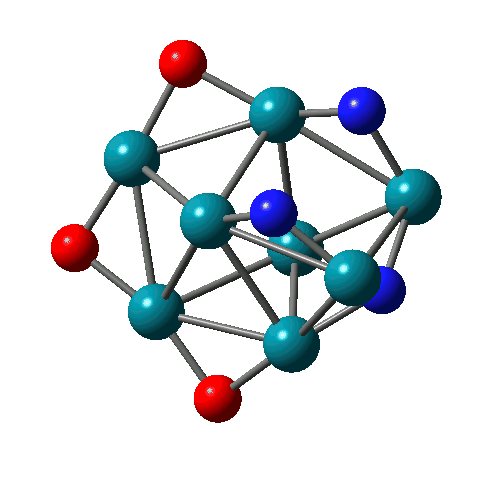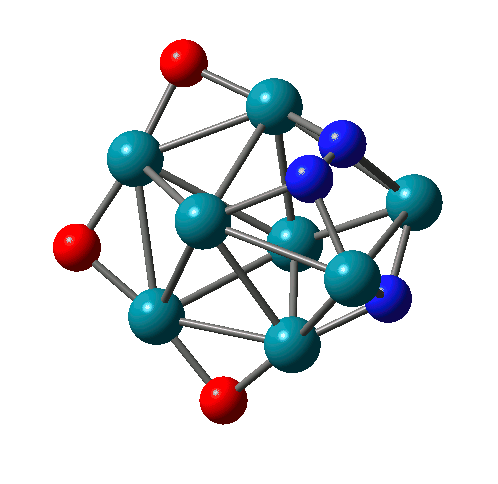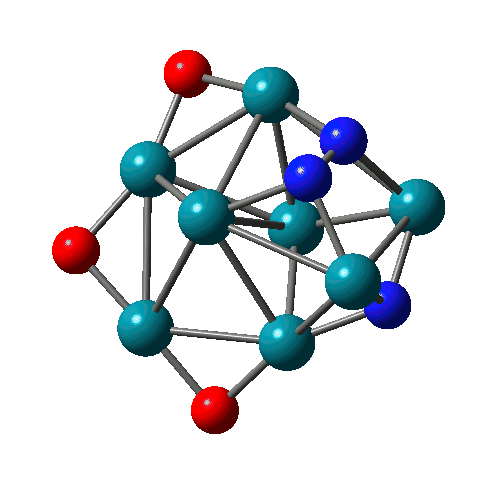 N-N bond formation on Rh8(NO)3+ |
2. Synthesis of novel nanomaterials by using laser
Nanomaterials are often synthesized as colloid in liquids. We have succeeded in developing a new method to produce stable noble metal nanoparticles in aqueous solution using pulsed lasers. We applied the laser ablation method, used for production of atoms and clusters in the gas phase, to the liquid phase. Laser ablation of metal plates in water produces stable nanoparticles for Au and Pt, even without any surfactants for stabilization. These methods have attracted worldwide attention; our paper published in 2000 has been cited more than 300 times in international journals, an international conference was held in Switzerland in 2010, and more than 100 papers have been submitted.
3. Structural analysis of clusters using infrared free electron laser
Radboud University in Nijmegen, the Netherlands, has an infrared (IR) free electron laser called "FELIX". FELIX can output high-intensity IR light over 100 cm-1, which is why researchers from all over the world come to this facility to use this light.
The IR laser is irradiated on clusters on which Ar atoms are attached as markers. When a vibrational frequency of the cluster matches the irradiated IR frequency, the cluster absorbs photons, and then the energy is converted into heat, resulting in desorption of the marker Ar atoms from the cluster. Therefore, we can know whether the cluster has absorbed the IR light for each wavenumber by monitoring the presence or absence of this desorption. The vibrational spectrum is thus obtained, and then we can determine the structure of the cluster because the vibrational spectrum can be regarded as the fingerprint of the cluster.
 FELIX building (from the website) |
 Inside |
 Cluster lab |
