FIRST ENERGETICS OF EXCITED-STATE AROMATICITY
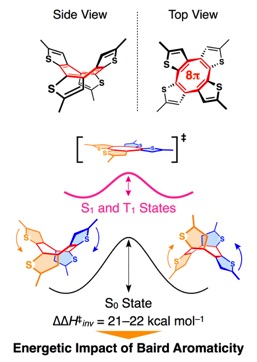
Aromaticity is one of the most important concepts in organic chemistry that can explain the stability of planar conformations found in cyclic π-conjugated molecules. In the electronic ground state, such molecules with 4n+2 π-electrons prefer to be planar (Hückel’s rule) because the resulting electronic conjugation leads to energetic stabilization. The stabilization effect of Hückel aromaticity has also been demonstrated. On the other hand, in the electronic excited state, cyclic π-conjugated molecules with 4n π-electrons prefer to be planar (Baird’s rule). However, the energetics of the Baird aromaticity has been elusive.
We, for the first time, substantiated the energetic impact of Baird aromaticity by investigating the ring inversion kinetics of several cyclic oligothiophenes that bear cyclic π-conjugated system with 4n π-electrons. This result will contribute to the progress of an exciting but much less explored area of organic photochemistry and related materials science.