Aspergillus oryzae is known to secrete many industrially useful enzymes and proteins, and hence is an attractive host for heterologous protein production. However, there is little knowledge on the secretory pathway machinery in fiamentous fungi including Aspergillus oryzae, in comparison to Saccharomyces cerevisiae. Our objective is to breed strains with high capability of enzyme secretion in Aspergillus oryzae, and in order to do this we are at present trying to visualize the protein secretory pathway structure.
Secretory protein after being translated in the ribosome, is transported into the endoplasmic reticulum, wherein proper folding of the protein occurs with the help of the chaperones, and other modifications such as glycosylation etc. take place. After proper modification the protein is transported into the Golgi body and finally secreted via a cell membrane.
BiP is one of the chaperones that is located in the endoplasmic reticulum, and coded by the bipA gene in Aspergillus oryzae. In order to visualize the endoplasmic reticulum, BipA-EGFP fusion protein was expressed in Aspergillus oryzae. When BipA-EGFP expression strains were observed under the fluorescence microscope, besides the annular structure of nuclear membrane, the fluorescence image resembling mesh-like stuructures was observed (Fig.1). A sequential observation revealed the active movement of this structure.
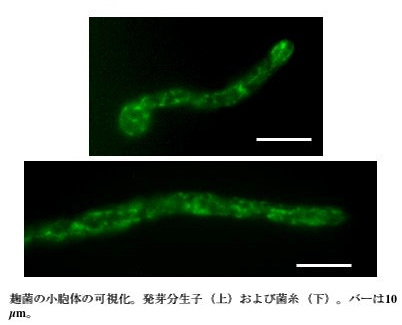
the upper ; a bud conidium.
the lower ; a hypha. The length of the bar is 10 μm.
Fig.1 Visualization of the endoplasmic reticulum in Aspergillus oryzae
Saccharomyces cerevisiae Sed5 is a t-SNARE protein and localizes on the Golgi membrane. By its specific binding with v-SNARE on a transport vesicle from an endoplasmic reticulum, a transport vesicle is docked on a Golgi body . When Aspergillus Sed5 homologue and EGFP were fused and expressed, dot-like fluorescence were observed (Fig.2). There has been no report on the observation of the Golgi bodies in filamentous fungi. This image resembles the Golgi bodies in yeasts or plants.
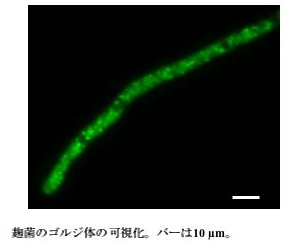
The length of the bar is 10 μm.
Fig.2 Visualization of Golgi bodies in Aspergillus oryzae
RNase T1 is a secretory enzyme of Aspergillus oryzae. We observed the localization of this enzyme by expression of RNase T1-EGFP fusion protein. RNase T1-EGFP fusion protein is secreted into the culture medium like other secretory enzymes. Upon fluorescence microscopy, EGFP fluorescence was distributed over the whole hyphae, and it was more brightly observed at the tip of hyphae (Fig.3). This observation suggested that secretory vesicle which transports RNase T1-EGFP fusion protein flows towards the tip cells of the growing hyphae. Furthermore, it supports the earlier opinion that enzymes such as amylase is secreted from the tips of the hyphae. On the other hand, when the basal cells of hyphae were observed, surprisingly much RNase T1-EGFP fusion protein localized on septum (Fig.4). This is an interesting result suggesting that there is secretory polarity not only in the tips of hyphae but also in the direction of septums. We think that RNase T1-EGFP fusion protein is a useful tool for the analysis of the secretory process in Aspergillus oryzae.
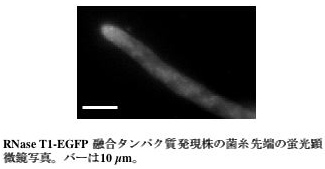
Green fluorescence at the hyphal can be observed
in the RNase T1-EGFP expression strain.
The length of the bar is 10 μm.
Fig.3
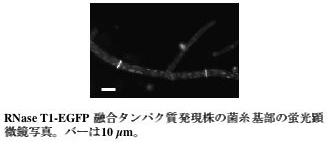
Green fluorescence at the septum in the basal cells of hyphae
can be observed in the RNase T1-EGFP fusion protein expression strain.
The length of the bar is 10 μm.
Fig.4